ISO 17025 writing services
2025-03-05 17:20ISO 17025 writing services
From Documentation to Certification – We Handle It All!
ISO/IEC 17025 Accreditation - Done for You
We write, review, and manage all ISO/IEC 17025 documents for your lab, ensuring a smooth path to accreditation. Unlimited requests, expert guidance, and fast delivery.
📌 Custom documents |
📌 Dedicated consultant |
📌 12-24h turnaround
👉 Get ISO 17025 Certified – Stress-Free!
Explore our services
🚀 Achieve ISO 17025 Compliance with Experts—Stress-Free!
Struggling with ISO/IEC 17025 compliance? Our expert consultants handle everything—from documentation to audit preparation. We craft custom procedures, quality manuals, training materials, and more, ensuring your lab meets all requirements.

Collaboration Space
Connect with your assigned consultant in a protected, centralized hub to collaborate more effectively.

Unlimited Requests
Get unlimited assistance from your dedicated consultant for any questions or requests you may have!

Quick and Reliable
With our 12-24 hours turnaround time you’ll have your requests when you need them.

Efficient workflows
Working with your consultant is an efficient and trustworthy process, ensuring that tasks are completed quickly and to the highest standard.

Seamless communication
Communicate with your team through chat messages, phone calls, emails and video meetings you can always stay connected!

Sheets and reports
Secure compliance with your procedures through regular monitoring. in your dedicated dashboard.

ISO/IEC17025 Compliance—Done for You by Experts!

ISO 17025 Complaince Portal with a dedicated consultant
Manage your ISO 17025 compliance like a PRO with QSE Academy , the All-in-One ISO 17025 compliance Portal that empowers collaboration with your team, and automates interaction with your consultant..

Dedicated full time ISO Consultant
At QSE Academy , we understand the importance of getting answers to your laboratory-critical questions quickly. That’s why we offer Unlimited Requests for consultancy or documents to all of our customers. With this feature, you can confidently ask us any question or request any document you need – from audit reports and compliance summaries....
Your ISO/IEC 17025 Compliance Hub – Manage, Train & Collaborate Effortlessly!
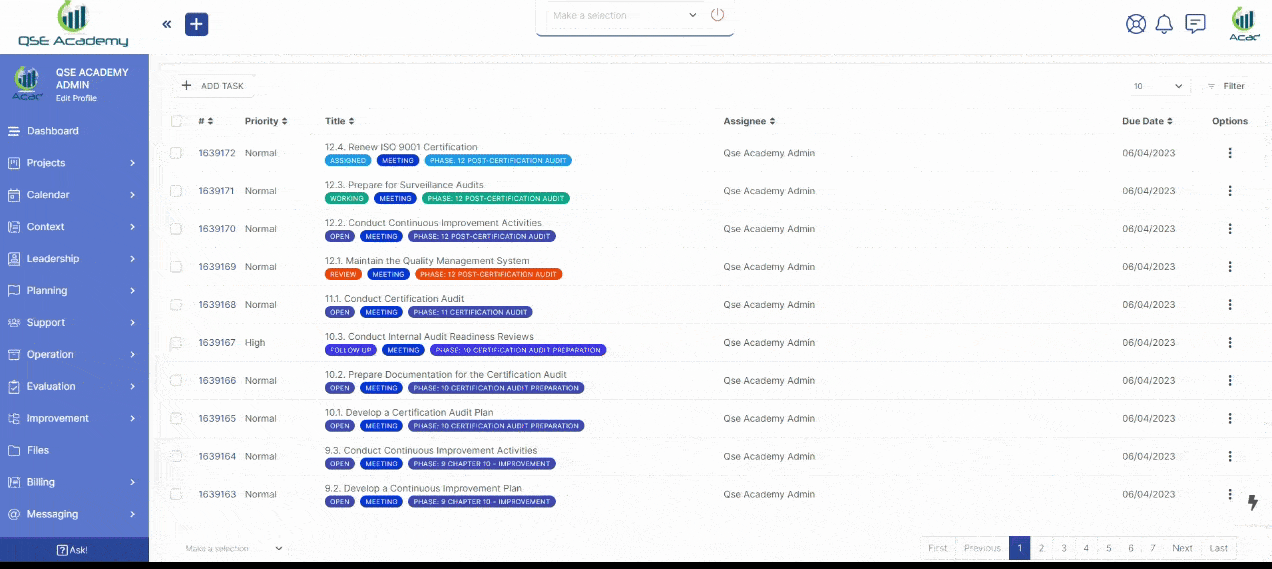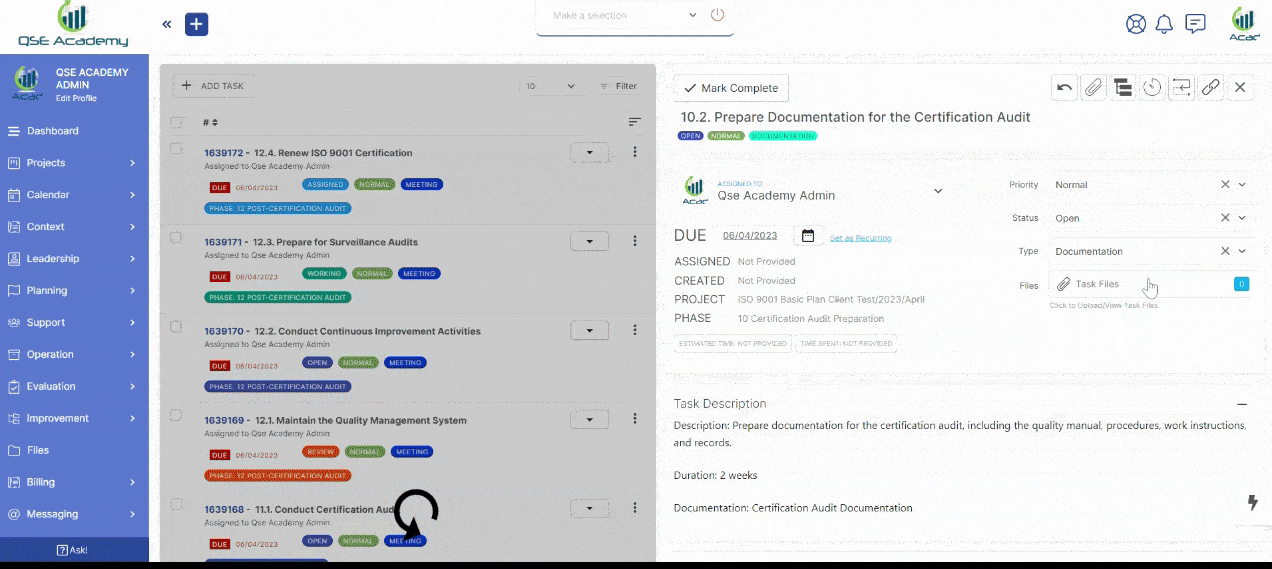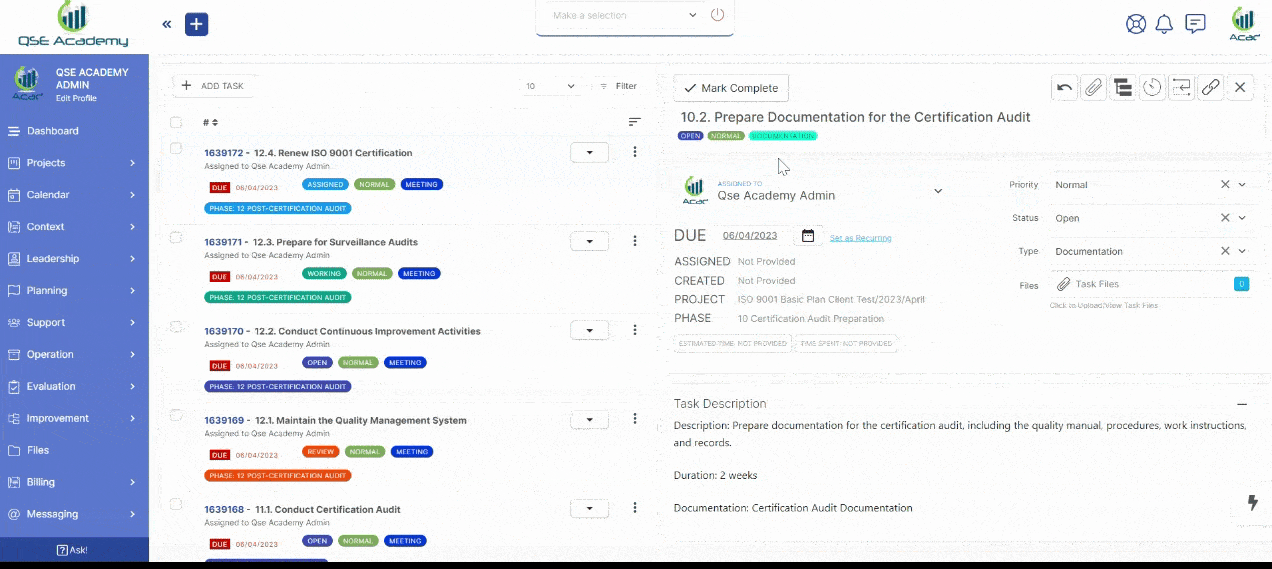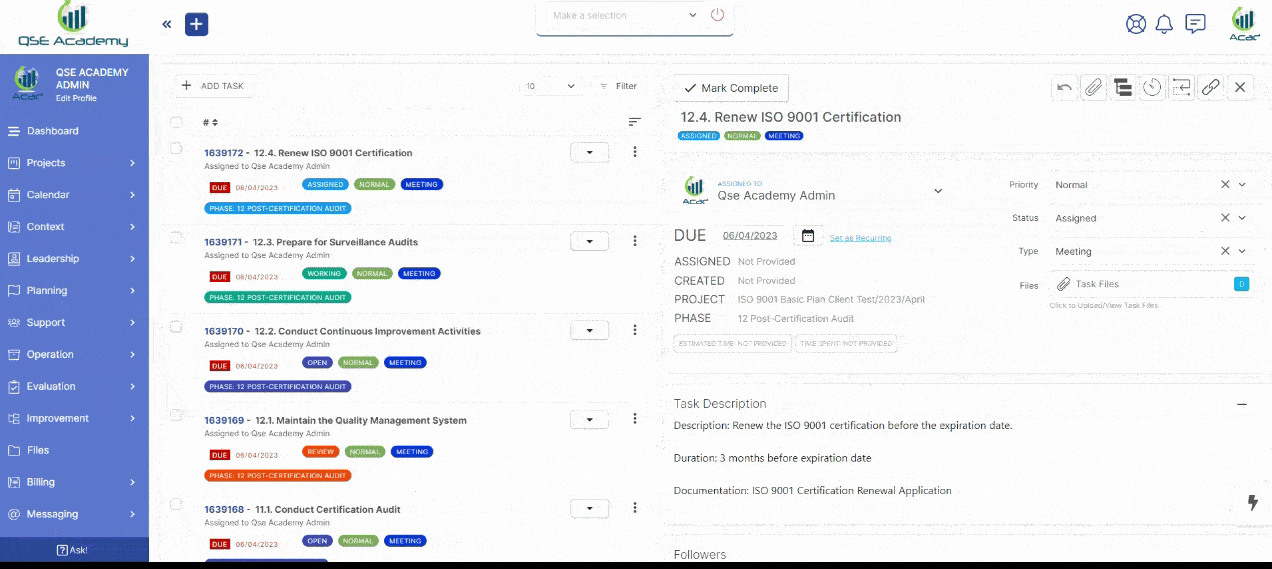
1. Work 1-on-1 with Your Consultant
We provide you with a dedicated Client Portal, where you can collaborate with your consultant, track progress, approve documents, and schedule meetings—all in one secure platform. No messy emails, no confusion—just a smooth, guided path to ISO 17025 accreditation.
2. Track Progress & Approve Documents
Stay in control with a clear roadmap and real-time updates on every step of your certification journey. Easily review, provide feedback, and approve documents with just a few clicks—ensuring a smooth and efficient process without delays.
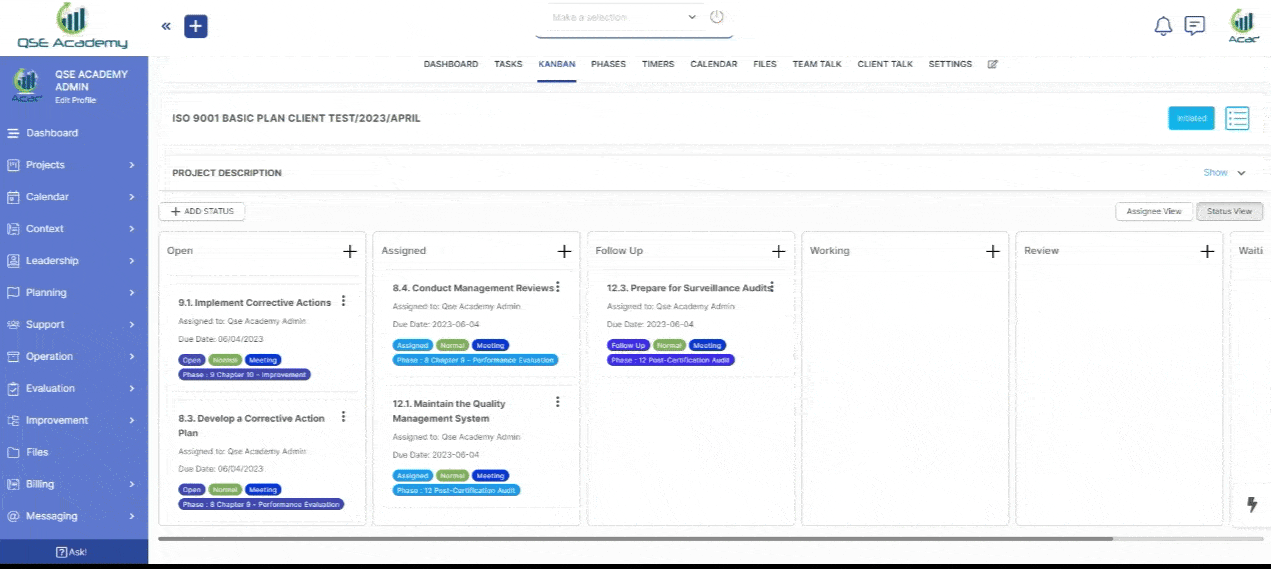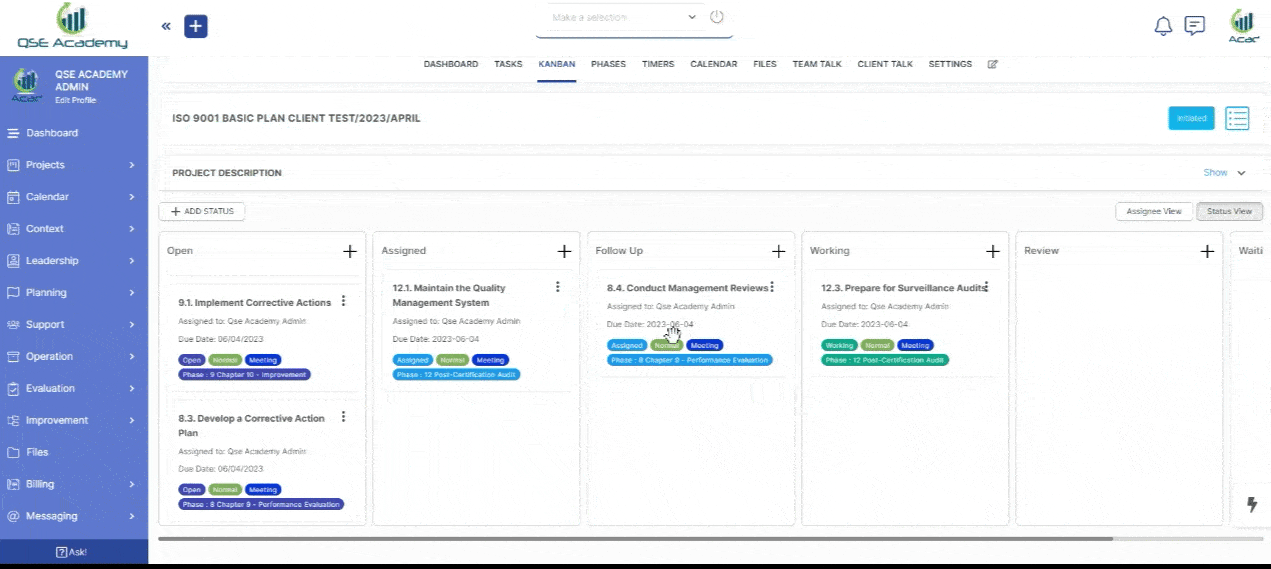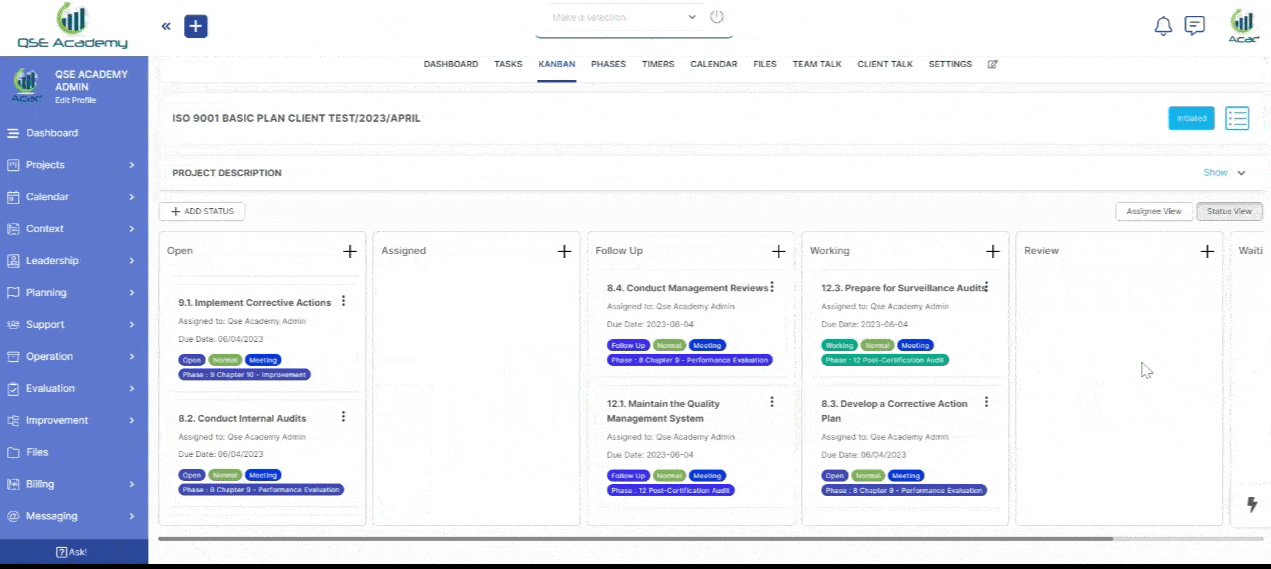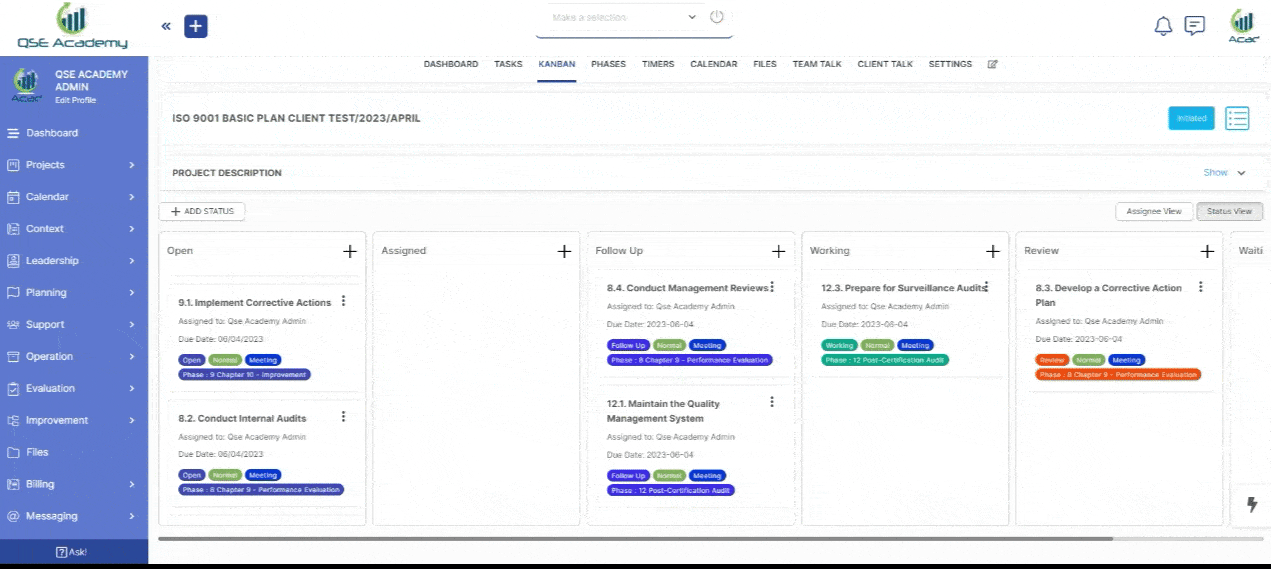
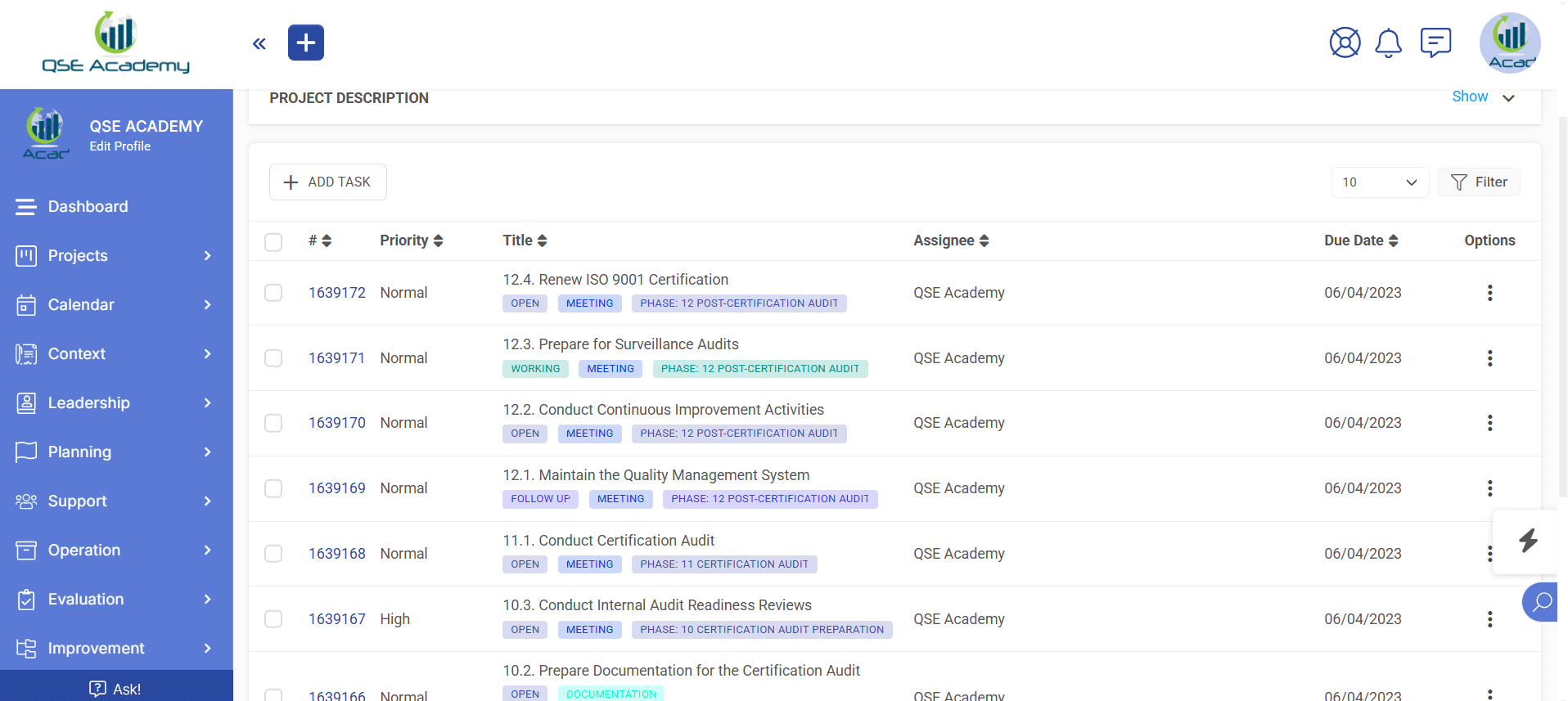
3. Schedule Meetings & Manage Tasks
Effortlessly book consultations, set reminders, and manage deadlines—all within your dedicated client portal. Stay on top of tasks, track upcoming meetings, and ensure every step of your certification process is completed on time, without the hassle of back-and-forth emails.
Our Team
Who We Are
We Have Only Best Skilled Professional Teams
Here at our organization, we only hire the very best experts in ISO/IEC 17025 standards - those who are unrivalled in the industry. Our dedicated team of experts understands the complexity and nuances of ISO 17025 and can help you implement it into your business or organization.
Implementation Project Plan
Tailored ISO/IEC 17025 2017 Implementation Project Plan
Achieving ISO/IEC 17025 accreditation is a significant milestone for any laboratory, signifying a commitment to quality and competence.
Our expert consultants are here to guide you through every step of the implementation process, from initial consultation and gap analysis to final assessment and accreditation.
With our comprehensive project plan, tailored training programs, and dedicated support, we ensure your laboratory meets all ISO/IEC 17025 requirements efficiently and effectively.
Partner with us to enhance your laboratory’s credibility, improve operational processes, and gain international recognition.
Let us help you achieve excellence in laboratory management.
Introduction: Consultant Engagement and Initial Training (Duration: 0.5 Month)
Introductory Tasks
1.1 Initial Consultation and Project Kick-off
Task: Schedule Initial Consultation
- Description: Arrange a meeting with the lead implementer and the consultant to discuss the project scope, objectives, timelines, and responsibilities.
- Documentation: Project plan and meeting agenda.
- Meeting: Initial consultation meeting.
Task: Conduct Gap Analysis
- Description: Perform a detailed gap analysis to identify the current state of the laboratory and areas needing improvement to meet ISO/IEC 17025 requirements.
- Documentation: Gap analysis report.
- Meeting: Meeting with the consultant to review gap analysis findings.
1.2 Training
Task: Develop Training Plan
- Description: Create a comprehensive training plan covering all aspects of ISO/IEC 17025 implementation.
- Documentation: Training plan document.
- Meeting: Meeting with the consultant to review and finalize the training plan.
Task: Conduct Staff Training
- Description: Provide training sessions for all relevant staff on ISO/IEC 17025 requirements and the implementation process.
- Documentation: Training materials and attendance records.
- Meeting: Training sessions with the consultant.
Section 1: General Requirements (Duration: 0.5 Month)
4.1 Impartiality
Task: Develop an Impartiality Policy
- Description: Create and document a policy ensuring that all laboratory activities are conducted impartially.
- Documentation: Impartiality policy document.
- Meeting: Initial meeting with the consultant to discuss and draft the policy.
Task: Conduct Risk Assessment for Impartiality
- Description: Identify and assess risks to impartiality in laboratory activities, and document mitigation strategies.
- Documentation: Risk assessment report.
- Meeting: Periodic meetings with the consultant to review identified risks and mitigation strategies.
Task: Implement Mitigation Measures
- Description: Apply and document measures to eliminate or minimize identified risks to impartiality.
- Documentation: Risk mitigation action plan.
- Meeting: Follow-up meeting with the consultant to evaluate the effectiveness of mitigation strategies.
4.2 Confidentiality
Task: Draft Confidentiality Agreements
- Description: Create legally binding confidentiality agreements for personnel and external parties.
- Documentation: Confidentiality agreements.
- Meeting: Consult with legal and compliance experts to finalize agreements.
Task: Establish Confidentiality Procedures
- Description: Develop procedures to manage information confidentiality in laboratory activities.
- Documentation: Confidentiality management procedures.
- Meeting: Training session with staff and consultant to ensure understanding and compliance.
Section 2: Structural Requirements (Duration: 0.5 Month)
5.1 Legal Entity
Task: Verify Legal Entity Status
- Description: Ensure the laboratory is a legal entity or part of a legal entity, and document the status.
- Documentation: Legal entity documentation (e.g., registration certificates).
- Meeting: Initial meeting with legal advisors to review and confirm the legal structure.
5.2 Management Responsibility
Task: Define Management Responsibilities
- Description: Identify and document the responsibilities of management personnel for laboratory activities.
- Documentation: Organizational chart and management responsibilities document.
- Meeting: Meeting with senior management to confirm responsibilities and authority.
5.3 Scope of Laboratory Activities
Task: Define Scope of Activities
- Description: Document the range of laboratory activities that conform to ISO/IEC 17025 requirements.
- Documentation: Scope of activities document.
- Meeting: Consultation with the consultant to review and validate the defined scope.
5.4 Organizational Structure
Task: Establish Organizational Structure
- Description: Define and document the organizational structure, including relationships between management, technical operations, and support services.
- Documentation: Organizational structure document.
- Meeting: Meeting with all relevant departments to communicate and validate the structure.
5.5 Resource Allocation
Task: Allocate Necessary Resources
- Description: Ensure the allocation of necessary resources (personnel, equipment, facilities) to laboratory activities.
- Documentation: Resource allocation plan.
- Meeting: Periodic meetings with the consultant to assess resource needs and adjustments.
Task: Monitor and Review Resources
- Description: Regularly monitor and review resource allocation to ensure ongoing compliance and effectiveness.
- Documentation: Resource review reports.
- Meeting: Quarterly review meetings with the consultant to discuss resource adjustments and improvements.
Section 3: Resource Requirements (Duration: 1.5 Months)
6.1 General
Task: Review and Identify Resource Requirements
- Description: Assess and document all necessary resources including personnel, facilities, equipment, systems, and support services.
- Documentation: Resource requirement document.
- Meeting: Schedule a meeting with the consultant to verify and validate resource requirements.
6.2 Personnel
Task: Define Competence Requirements
- Description: Document the competence requirements for each function influencing laboratory activities, including education, qualification, training, technical knowledge, skills, and experience.
- Documentation: Competence requirements matrix.
- Meeting: Initial meeting with HR and the consultant to discuss competence criteria.
Task: Develop Training Programs
- Description: Develop and implement training programs to ensure personnel are competent to perform assigned tasks.
- Documentation: Training program documents.
- Meeting: Training session with the consultant to guide staff through the new training programs.
Task: Authorize Personnel
- Description: Formally authorize personnel to perform specific laboratory activities.
- Documentation: Authorization records.
- Meeting: Internal review meeting to approve personnel authorizations.
6.3 Facilities and Environmental Conditions
Task: Assess Facility Requirements
- Description: Evaluate and document the facility and environmental conditions necessary for laboratory activities.
- Documentation: Facility assessment report.
- Meeting: Consultation with the consultant to review facility requirements.
Task: Monitor Environmental Conditions
- Description: Implement a system to monitor, control, and record environmental conditions.
- Documentation: Environmental monitoring logs.
- Meeting: Regular review meetings to ensure compliance with environmental control measures.
6.4 Equipment
Task: Inventory and Assess Equipment
- Description: Create an inventory of all equipment and assess its suitability for laboratory activities.
- Documentation: Equipment inventory list.
- Meeting: Review meeting with the consultant to verify equipment suitability.
Task: Implement Equipment Maintenance Procedures
- Description: Develop and document procedures for handling, transport, storage, use, and planned maintenance of equipment.
- Documentation: Equipment maintenance procedures.
- Meeting: Training session with the consultant to ensure all personnel are aware of equipment handling procedures.
6.5 Metrological Traceability
Task: Establish Calibration Program
- Description: Develop a calibration program to ensure the metrological traceability of measurement results.
- Documentation: Calibration program document.
- Meeting: Periodic review meetings with the consultant to assess the effectiveness of the calibration program.
6.6 Externally Provided Products and Services
Task: Evaluate Suppliers
- Description: Assess and approve suppliers of externally provided products and services to ensure they meet laboratory requirements.
- Documentation: Approved supplier list.
- Meeting: Initial and periodic meetings with the consultant to review supplier evaluations.
Section 4: Process Requirements (Duration: 2 Months)
7.1 Review of Requests, Tenders, and Contracts
Task: Develop Review Procedures
- Description: Create procedures to review requests, tenders, and contracts to ensure the laboratory has the capability to meet requirements.
- Documentation: Review procedure document.
- Meeting: Training session with staff to ensure understanding of review procedures.
7.2 Selection, Verification, and Validation of Methods
Task: Document Method Selection Procedures
- Description: Establish procedures for the selection and verification of methods used in laboratory activities.
- Documentation: Method selection procedures.
- Meeting: Review meeting with the consultant to validate method selection procedures
Task: Validate Methods
- Description: Perform validation of methods to ensure they are fit for intended use.
- Documentation: Method validation reports.
- Meeting: Periodic meetings to review validation results and approve methods.
7.3 Sampling
Task: Develop Sampling Plans
- Description: Create sampling plans and methods to ensure the validity of subsequent testing or calibration results.
- Documentation: Sampling plans and methods.
- Meeting: Review sampling plans with the consultant to ensure they meet laboratory requirements.
7.4 Handling of Test or Calibration Items
Task: Establish Handling Procedures
- Description: Develop procedures for the transportation, receipt, handling, protection, storage, retention, and disposal or return of test or calibration items.
- Documentation: Handling procedures document.
- Meeting: Training session with staff to ensure proper handling of items.
7.5 Technical Records
Task: Implement Record Keeping System
- Description: Create and maintain a system for managing technical records, including procedures for record retention and retrieval.
- Documentation: Technical records management procedures.
- Meeting: Internal meeting to train staff on the new record-keeping system.
7.6 Evaluation of Measurement Uncertainty
Task: Establish Measurement Uncertainty Evaluation Procedures
- Description: Develop procedures to evaluate measurement uncertainty for relevant laboratory activities.
- Documentation: Measurement uncertainty evaluation procedures.
- Meeting: Review meeting with the consultant to validate and approve the evaluation procedures.
7.7 Ensuring the Validity of Results
Task: Implement Quality Control Procedures
- Description: Establish procedures for ensuring the validity of results, including the use of control charts, proficiency testing, and inter-laboratory comparisons.
- Documentation: Quality control procedures document.
- Meeting: Regular review meetings with the consultant to discuss quality control measures.
7.8 Reporting of Results
Task: Develop Reporting Procedures
- Description: Create procedures for reporting test and calibration results, ensuring they meet customer and regulatory requirements.
- Documentation: Reporting procedures document.
- Meeting: Training session with staff to ensure understanding of reporting procedures.
7.9 Complaints
Task: Establish Complaints Handling Procedures
- Description: Develop and document procedures for handling complaints from customers and other stakeholders.
- Documentation: Complaints handling procedures.
- Meeting: Periodic review meetings with the consultant to assess the effectiveness of the complaints handling process.
7.10 Nonconforming Work
Task: Develop Procedures for Nonconforming Work
- Description: Create procedures to manage nonconforming work, including identification, documentation, evaluation, and corrective action.
- Documentation: Nonconforming work procedures.
- Meeting: Training session with staff to ensure understanding and compliance.
- 7.11 Control of Data and Information Management
Task: Implement Data Management System
- Description: Establish a system for managing data and information, ensuring accuracy, security, and confidentiality. –
- Documentation: Data management procedures. –
- Meeting: Training session with staff to ensure proper data management practices.
Section 5: Management System Requirements (Duration: 1.5 Months)
8.1 Options
Task: Choose Management System Option
- Description: Decide whether to adopt Option A or Option B for the management system.
- Documentation: Decision document.
- Meeting: Meeting with the consultant to discuss options and make a decision.
8.2 Management System Documentation
Task: Document Management System
- Description: Develop and maintain documentation for the management system, including policies, procedures, and records.
- Documentation: Management system documentation.
- Meeting: Review meeting with the consultant to ensure completeness and compliance.
8.3 Control of Management System Documents
Task: Implement Document Control Procedures
- Description: Establish procedures for controlling management system documents, ensuring they are reviewed, approved, and accessible.
- Documentation: Document control procedures.
- Meeting: Training session with staff to ensure proper document control.
8.4 Control of Records
Task: Develop Record Control Procedures
- Description: Create procedures for controlling records, ensuring they are maintained, protected, and retrievable.
- Documentation: Record control procedures.
- Meeting: Internal meeting to train staff on record control procedures.
8.5 Actions to Address Risks and Opportunities
Task: Conduct Risk and Opportunity Assessment
- Description: Identify and assess risks and opportunities related to the laboratory’s activities, and develop action plans.
- Documentation: Risk and opportunity assessment report.
- Meeting: Regular review meetings with the consultant to discuss and update action plans.
8.6 Improvement
Task: Implement Improvement Actions
- Description: Develop and document actions to improve the effectiveness of the management system.
- Documentation: Improvement action plan.
- Meeting: Periodic meetings to review progress and effectiveness of improvement actions.
8.7 Corrective Actions
Task: Establish Corrective Action Procedures
- Description: Create procedures to identify, document, and address nonconformities and implement corrective actions.
- Documentation: Corrective action procedures.
- Meeting: Training session with staff to ensure proper implementation of corrective actions.
8.8 Internal Audits
Task: Plan and Conduct Internal Audits
- Description: Develop an internal audit program to evaluate the effectiveness of the management system.
- Documentation: Internal audit plan and reports.
- Meeting: Audit planning and review meetings with the consultant.
8.9 Management Reviews
Task: Conduct Management Reviews
- Description: Plan and conduct management reviews to ensure the continuing suitability, adequacy, and effectiveness of the management system.
- Documentation: Management review meeting minutes and reports.
- Meeting: Periodic management review meetings.
Final Assessment and Accreditation Preparation (Duration: 1 Month)
9.1 Final Assessment Preparation
Task: Conduct Final Pre-Assessment
- Description: Perform a final assessment to ensure all ISO/IEC 17025 requirements are met and identify any remaining gaps.
- Documentation: Pre-assessment report.
- Meeting: Meeting with the consultant to review the final assessment findings.
9.2 Internal Audit
Task: Conduct Internal Audit
- Description: Perform a comprehensive internal audit to verify compliance with ISO/IEC 17025 standards.
- Documentation: Internal audit report.
- Meeting: Post-audit meeting with the consultant to discuss findings and corrective actions.
9.3 Management Review Preparation
Task: Prepare for Management Review
- Description: Organize and document the management review process, ensuring all aspects of the management system are evaluated.
- Documentation: Management review documents.
- Meeting: Management review meeting to finalize the review process.
9.4 Accreditation Body Selection 4.
Task: Select Accreditation Body
- Description: Research and select a suitable accreditation body for ISO/IEC 17025 certification.
- Documentation: Accreditation body selection report.
- Meeting: Consultation with the consultant to finalize the selection of the accreditation body.
These tasks ensure a thorough and organized approach to implementing ISO/IEC 17025 2017, promoting competence, impartiality, and consistent operation within the laboratory.
FAQ
Is this service able to help a new laboratory achieve ISO 17025 accreditation?
Yes! This is not a consultancy—it’s a done-for-you solution where we handle everything for you. We don’t just advise; we write the documents, manage the process, and ensure your lab meets all ISO 17025 requirements. You get expert-crafted documentation, structured guidance, and full support until accreditation—without the hassle of doing it yourself.
What does unlimited requests mean?
Unlimited requests means you can ask for any type of documentation related to your laboratory and ISO 17025 accreditation. Whether you need procedures, quality manuals, forms, checklists, training materials, or audit documentation, we create and customize them for you—as many as you need, with no limits.
What types of documents are included in the service?
- Quality manuals
- Standard operating procedures (SOPs)
- Forms and checklists
- Training materials
- Risk assessments
- Internal audit reports
Every document is tailored to your laboratory’s needs to ensure full compliance with ISO/IEC 17025.
When can I expect to receive a response for my inquires?
You can expect a response to your inquiries within 12 to 24 hours. Our team ensures timely support to keep your ISO 17025 accreditation process on track.
Do I have to sign a long-term contract?
No, there is no long-term contract. This is a one-time payment service with no hidden fees or recurring charges. Plus, we offer a 30-day money-back guarantee for your peace of mind.
How does the 30-day money back guarantee work?
Our 30-day money-back guarantee is simple: we work together for 30 days, and if you’re not satisfied with our work, you’ll receive a full refund—no questions asked. This ensures you can move forward with confidence, knowing there’s no risk.

Choose the Perfect Plan for Your
ISO/IEC 17025 Accreditation Journey
We offer simple, upfront pricing for ISO/IEC 17025 accreditation with no hidden costs. Choose the plan that fits your laboratory size—whether you're a small lab (1-10 personnel) or a growing facility (20-50 personnel). Our guided accreditation system ensures a smooth and efficient process—guaranteed, or you don’t pay.
Small Laboratory Plan (1-10 Employees)
- ✅ Guaranteed Accreditation– Or You Don’t Pay
- ✅ Done-for-You ISO/IEC 17025 Documentation
- ✅ 1-on-1 Consultant Support
- ✅ Pre-Audit Readiness Assessment
- ✅ Internal Audit & Compliance Check
- ✅ Audit-Ready Compliance Check
- ✅ Access to Your Client Portal
- ✅ Pre-Audit & Accreditation Audit Support
- ✅ Fast-Track Accreditation in 180 Days
$ 4500
Growing Labs Plan (20-50 Employees)
- ✅ Everything in the Small Labs Plan
- ✅ Extended 1-on-1 Support for Larger Teams
- ✅ Additional Training for Key Staff
- ✅ Custom Process Optimization
- ✅ Priority Audit Preparation & Review
$8000
Trusted by Organizations Worldwide
"See what our satisfied customers have to say about their experience with QSE Academy ISO 9001 certification system
ISO/IEC 17025 Accreditation – Guaranteed Quality & No-Risk Commitment
We are committed to delivering ISO/IEC 17025 accreditation success, and we stand behind our process with a risk-free guarantee.
🔹 30-Day Satisfaction Guarantee – We Work, You Decide
We invest significant time and expertise upfront to craft custom documentation, guide your lab, and manage your accreditation process. If, after 30 days of working together, you’re not satisfied with our work, we’ll issue a full refund—no questions asked. However, we don’t accept charity—this is for those serious about achieving accreditation.
🔹 Our Guarantee: We Work Until You Succeed
If your lab follows our process but doesn’t meet accreditation requirements on the first attempt, we continue working with you at no extra cost until you achieve compliance.
🔹 Transparent Pricing, No Hidden Fees
Our pricing is clear and upfront, with no unexpected costs. You know exactly what you’re paying for before we begin.
With our expert guidance, structured process, and commitment to results, we ensure your ISO/IEC 17025 accreditation—without risk or hassle.
Contact us
Have more questions? Book Your Free 30-Minute Consultation!
Struggling with ISO/IEC 17025 accreditation? Not sure where to start or what’s missing in your compliance process?
Our team is offering a free 30-minute strategy call to help you:
✅ Assess your laboratory’s current compliance status and identify key gaps.
✅ Understand ISO/IEC 17025 requirements and what they mean for your lab.
✅ Get a clear, actionable roadmap to move forward with accreditation.
📅 Limited slots available each week – Book your free consultation now and take the next step toward ISO/IEC 17025 compliance!


