ISO 22716 services
2025-03-05 21:57ISO 22716 services
Welcome to QSE Academy
Achieve ISO 22716 Certification – Done-for-You Compliance Comprehensive Documentation Training Modules Dedicated Consultant
Struggling with ISO 22716 implementation? We handle everything for you—from documentation to full compliance—so you can focus on your business.
Our all-in-one solution includes:
✅ Custom ISO 22716 documentation tailored to your operations
✅ Expert guidance to ensure full compliance with GMP requirements
✅ Step-by-step support until certification
Explore our services
🚀 Achieve ISO 22716 Compliance with Experts—Stress-Free!
Struggling with ISO 22716 (GMP) compliance? Our expert team handles everything—from documentation to certification preparation. We craft custom procedures, quality manuals, training materials, and more, ensuring your business meets all Good Manufacturing Practices (GMP) requirements with ease.

Collaboration Space
Connect with your assigned consultant in a protected, centralized hub to collaborate more effectively.

Unlimited Requests
Get unlimited assistance from your dedicated consultant for any questions or requests you may have!

Quick and Reliable
With our 12-24 hours turnaround time you’ll have your requests when you need them.

Efficient workflows
Working with your consultant is an efficient and trustworthy process, ensuring that tasks are completed quickly and to the highest standard.

Seamless communication
Communicate with your team through chat messages, phone calls, emails and video meetings you can always stay connected!

Sheets and reports
Secure compliance with your procedures through regular monitoring. in your dedicated dashboard.

ISO 22716 Compliance—Done for You by Experts!

ISO 22716 Compliance Portal with a Dedicated Consultant
Manage your ISO 22716 compliance like a pro with QSE Academy—the all-in-one ISO 22716 compliance portal that empowers collaboration with your team and automates interaction with your consultant. Simplify your GMP certification process with expert guidance at every step.

Dedicated full time ISO Consultant
At QSE Academy, we understand the importance of getting fast and accurate answers to your ISO 22716 compliance questions. That’s why we offer Unlimited Requests for consultancy and documentation to all our customers. You can confidently ask us any question or request any document—from audit reports to compliance summaries—ensuring your business stays on track for GMP certification.
Your ISO 22716 Compliance Hub – Manage, Train & Collaborate Effortlessly!
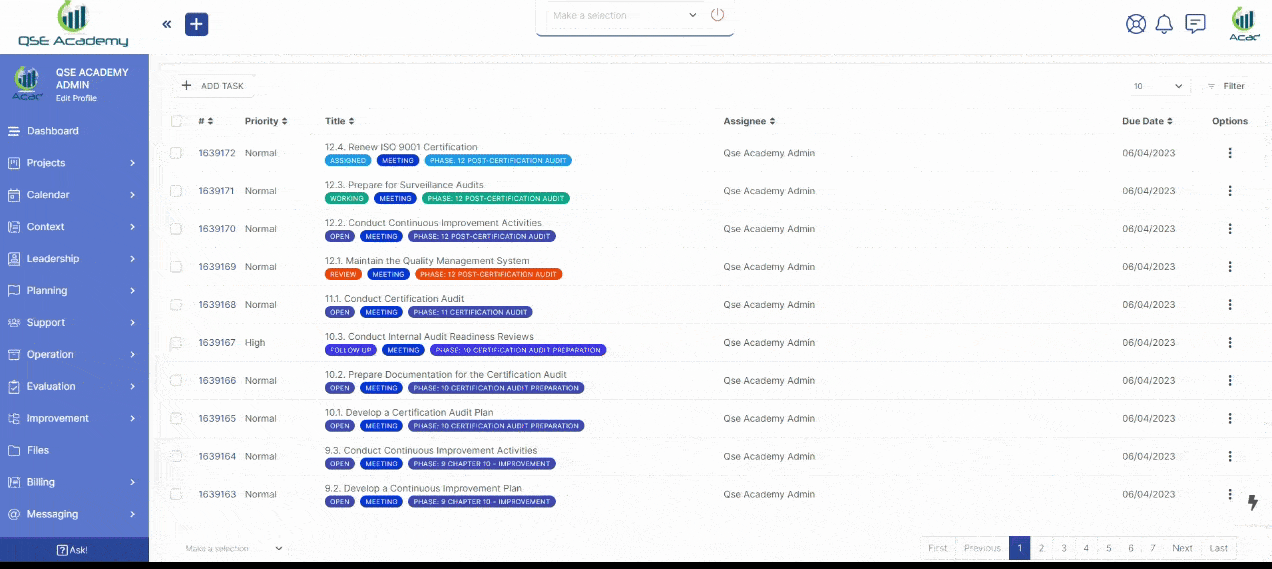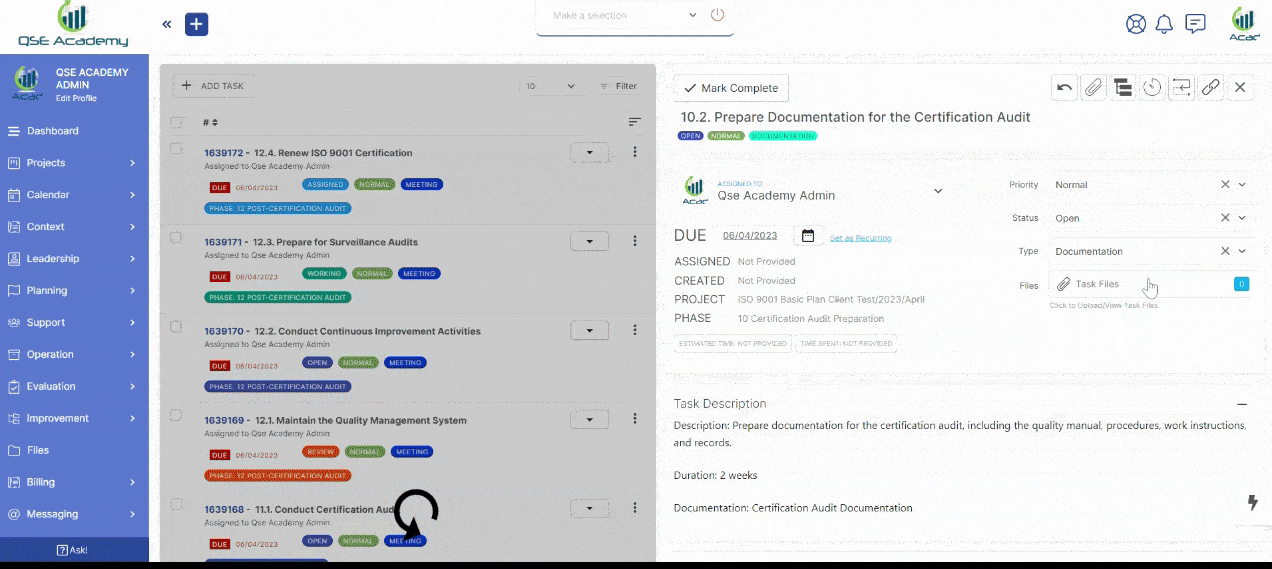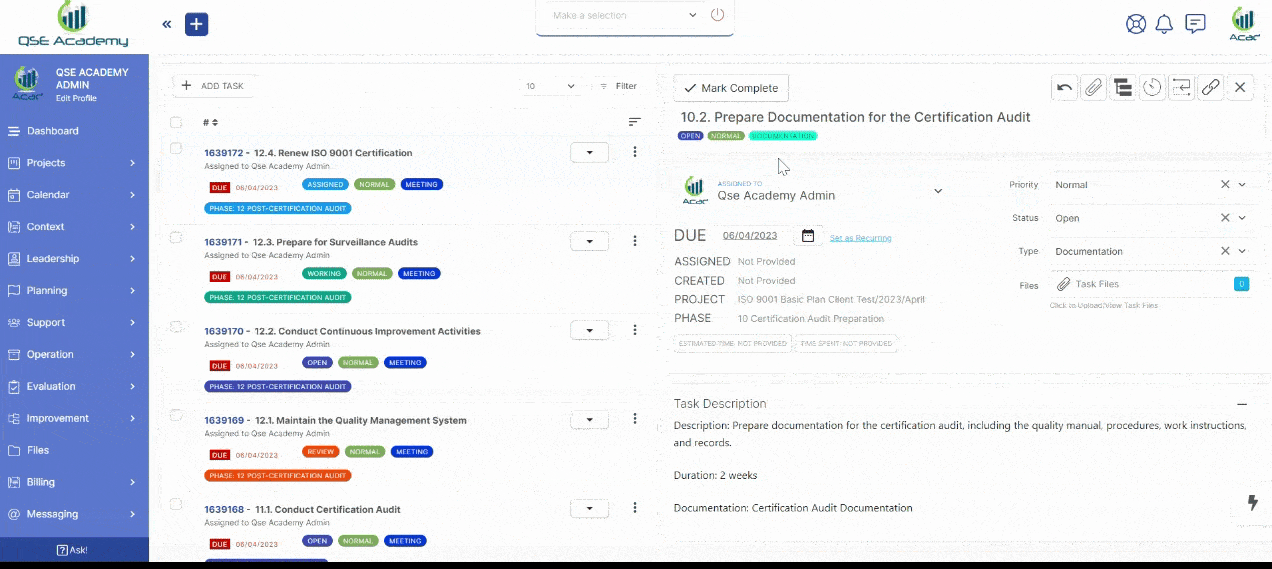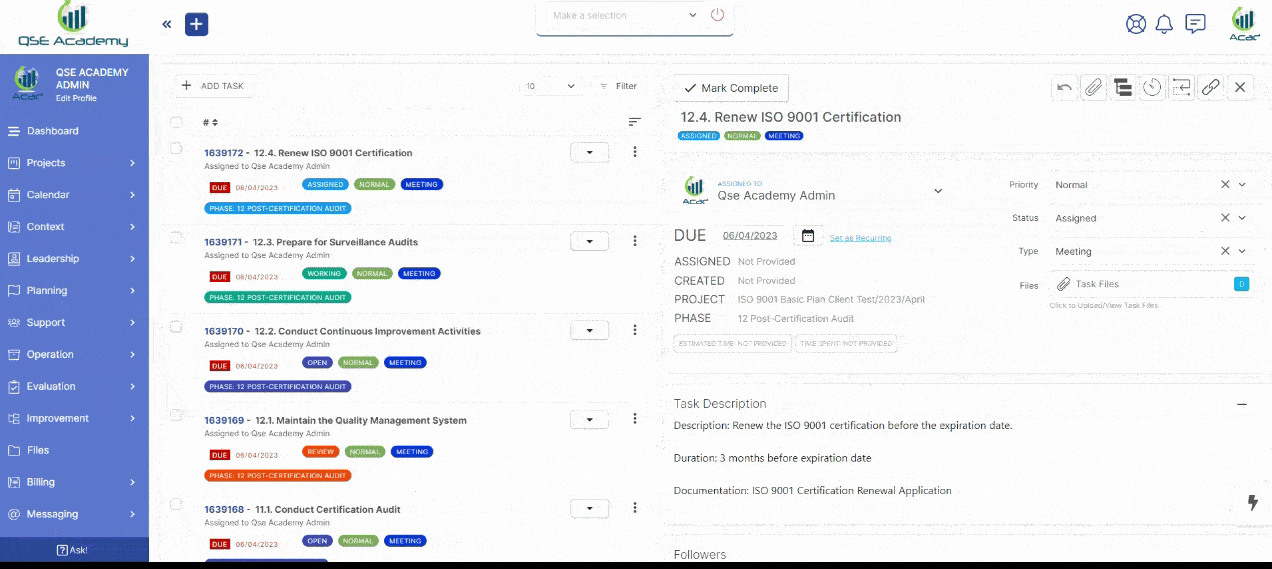
1. Work 1-on-1 with Your Consultant
We provide you with a dedicated Client Portal, where you can collaborate with your consultant, track progress, approve documents, and schedule meetings—all in one secure platform. No messy emails, no confusion—just a smooth, guided path to ISO 22716 (GMP) certification.
2. Track Progress & Approve Documents
Stay in control with a clear roadmap and real-time updates on every step of your ISO 22716 (GMP) certification journey. Easily review, provide feedback, and approve documents with just a few clicks—ensuring a smooth, efficient, and delay-free process.
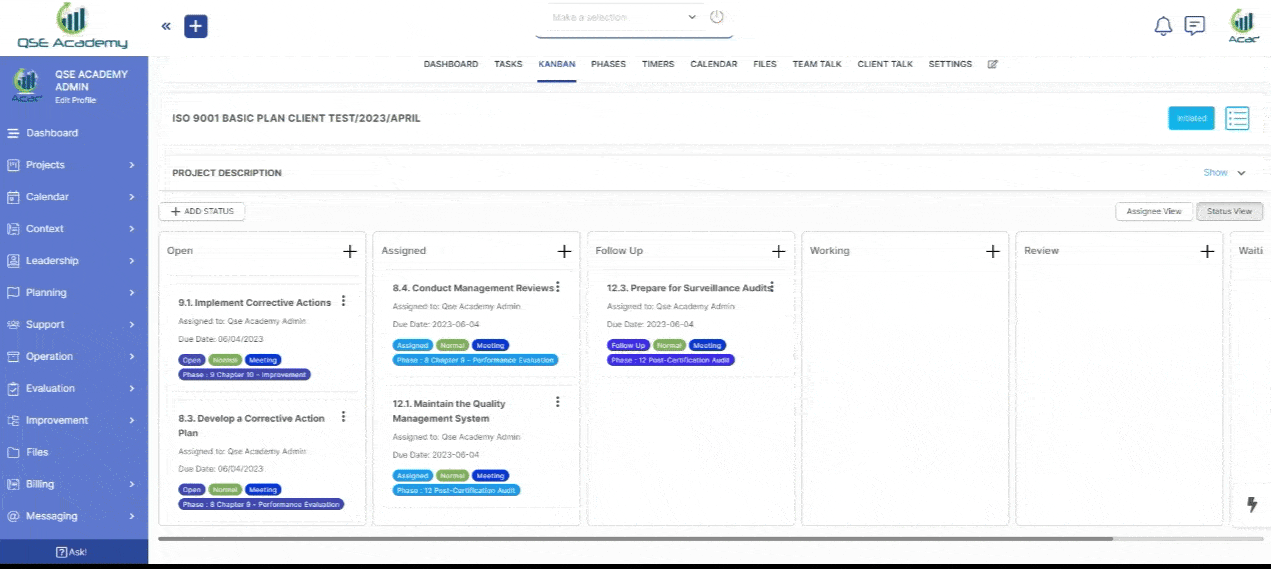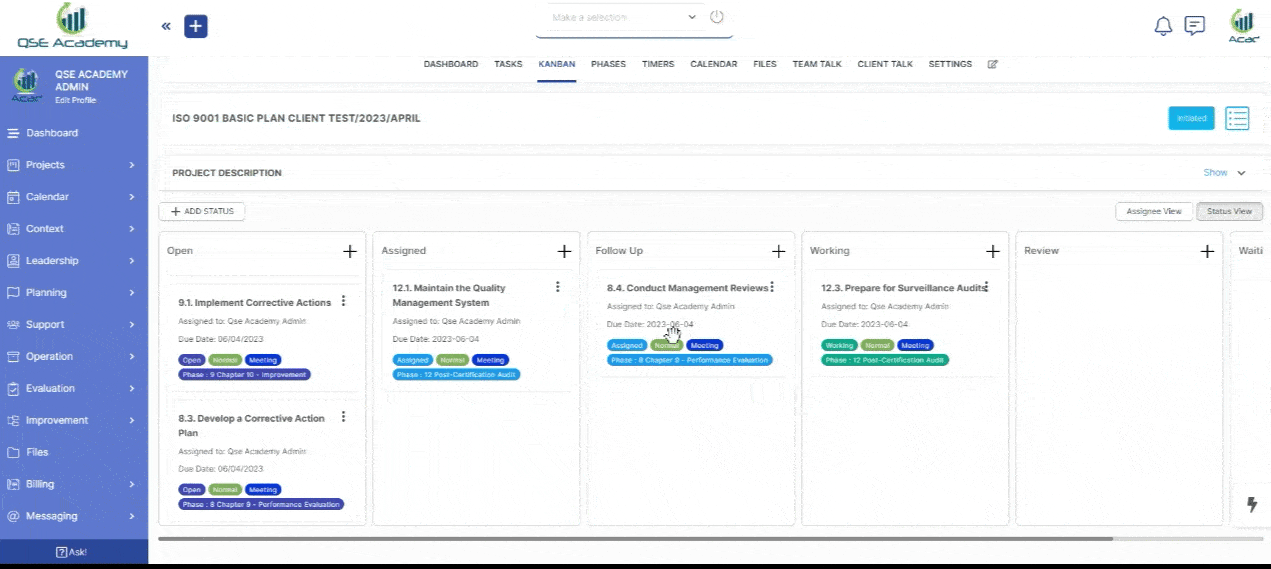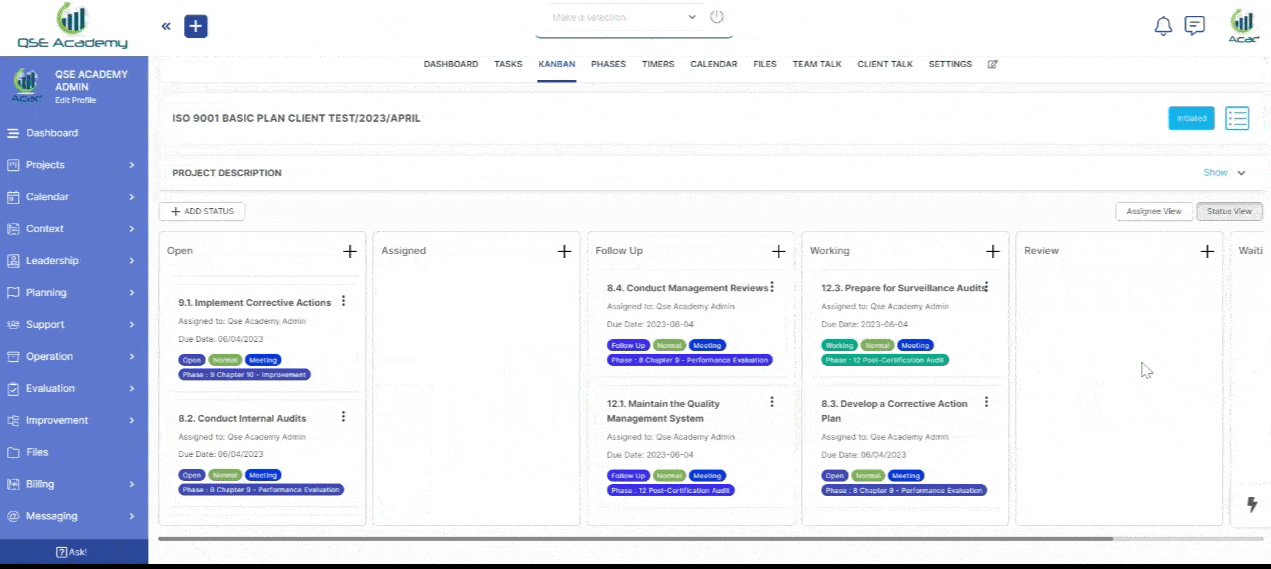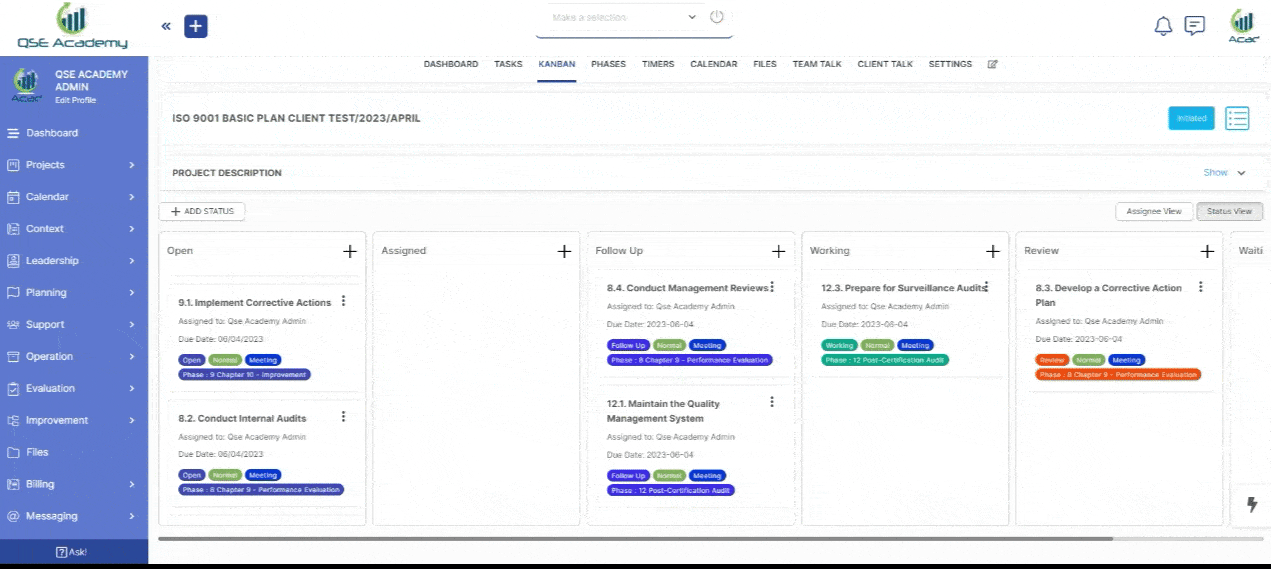
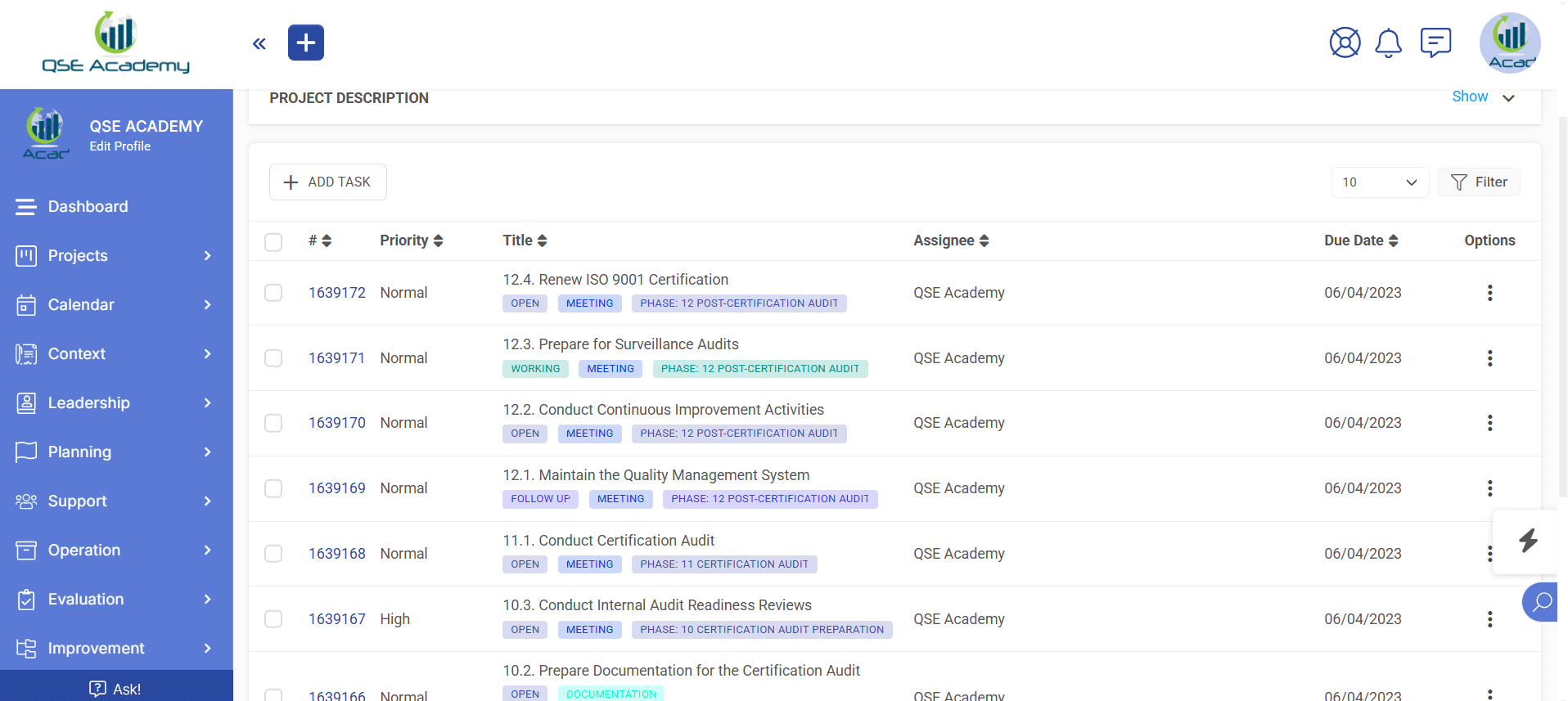
3. Schedule Meetings & Manage Tasks
Stay in control with a clear roadmap and real-time updates on every step of your ISO 22716 (GMP) certification journey. Easily review, provide feedback, and approve documents with just a few clicks—ensuring a smooth, efficient, and delay-free process
Our Team
Who We Are
We Have Only Best Skilled Professional Teams
Here at our organization, we only hire the very best experts in ISO 22716 (GMP) standards—professionals who are unrivaled in the industry. Our dedicated team understands the complexity and nuances of ISO 22716 and will help you implement it seamlessly into your business, ensuring full compliance with Good Manufacturing Practices (GMP).
Implementation Project Plan
Total Implementation Duration: 6 Months
ISO 22716 Implementation Project Plan
Achieving ISO 22716 compliance demonstrates your commitment to implementing Good Manufacturing Practices (GMP) in the cosmetic industry. This ensures product safety, quality, and regulatory compliance. Our expert consultants guide you through each step, providing tailored solutions, training, and ongoing support. This plan outlines a structured approach to meet ISO 22716 requirements effectively and efficiently.
Introduction: Consultant Engagement and Initial Training (Duration: 0.5 Month)
Introductory Tasks
1.1 Initial Consultation and Project Kick-off
Task: Schedule Initial Consultation
- Description: Arrange a meeting to discuss project scope, objectives, and timelines.
- Documentation: Project plan and meeting agenda.
- Meeting: Initial consultation meeting.
Task: Conduct Gap Analysis
- Description: Analyze existing practices against ISO 22716 requirements to identify gaps.
- Documentation: Gap analysis report.
- Meeting: Review findings with the consultant.
Task: Develop Training Plan
- Description: Create a comprehensive training plan on ISO 22716 requirements.
- Documentation: Training plan document.
- Meeting: Finalize the plan with the consultant.
- Description: Train personnel on GMP principles and implementation steps.
- Documentation: Training materials and attendance records.
- Meeting: Training sessions with all staff.
Section 1: General Requirements (Duration: 0.5 Month)
2.1 Quality Management System (QMS)
Task: Establish QMS
- Description: Develop a QMS tailored to ISO 22716 requirements.
- Documentation: QMS manual.
- Meeting: Review session with stakeholders.
2.2 Roles and Responsibilities
Task: Define Roles and Responsibilities
- Description: Identify and document personnel responsibilities for GMP compliance.
- Documentation: Organizational chart and role descriptions.
- Meeting: Confirmation meeting with management.
Section 2: Premises and Equipment (Duration: 1 Month)
3.1 Premises Layout and Design
Task: Review and Optimize Layout
- Description: Assess facility layout for compliance with GMP requirements.
- Documentation: Facility layout plan.
- Meeting: Site walkthrough with consultant.
3.2 Equipment Management
Task: Develop Equipment Maintenance Plan
- Description: Create procedures for equipment maintenance, calibration, and cleaning.
- Documentation: Maintenance plan.
- Meeting: Training on equipment protocols.
Section 3: Raw Materials and Packaging(Duration: 1.5 Months)
4.1 Supplier Management
Task: Evaluate and Approve Suppliers
- Description: Establish criteria for supplier evaluation and approval.
- Documentation: Approved supplier list.
- Meeting: Review supplier audits with the consultant.
4.2 Raw Material Control
Task: Implement Material Receipt Procedures
- Description: Develop procedures for the inspection and storage of raw materials.
- Documentation: Material control procedures.
- Meeting: Training sessions for staff.
Section 4: Production and Quality Control)(Duration: 2 Months)
5.1 Production Processes
Task: Document Production Procedures
- Description: Develop standard operating procedures (SOPs) for production.
- Documentation: SOPs for all processes.
- Meeting: Review production SOPs with the consultant.
5.2 Batch Records
Task: Implement Batch Record System
- Description: Establish a system to document and track production batches.
- Documentation: Batch record templates.
- Meeting: Staff training on record-keeping.
5.3 Quality Control
Task: Develop Quality Testing Protocols
- Description: Create protocols for testing raw materials, in-process products, and finished goods.
- Documentation: Testing protocols and logs.
- Meeting: Training with the quality team.
Section 5: Storage and Distribution (Duration: 1 Month)
6.1 Storage Conditions
Task: Define Storage Requirements
- Description: Identify appropriate storage conditions for raw materials and products.
- Documentation: Storage guidelines.
- Meeting: Staff training on storage protocols.
6.2 Distribution Controls
Task: Establish Distribution Procedures
- Description: Develop procedures for product distribution, ensuring traceability and quality.
- Documentation: Distribution records.
- Meeting: Training on distribution protocols.
Section 6:Documentation and Record-Keeping (Duration: 1.5 Months)
7.1 Document Control
Task: Implement Document Control System
- Description: Develop procedures for creating, reviewing, and approving documents.
- Documentation: Document control procedures.
- Meeting: Training session on document management.
7.2 Record Retention
Task: Define Record Retention Policies
- Description: Establish retention periods for records as per GMP requirements.
- Documentation: Retention policy document.
- Meeting: Internal review with staff.
Final Assessment and Certification Preparation(Duration: 1 Month)
8.1 Pre-Assessment Audit
Task: Conduct Pre-Assessment Audit
- Description: Review implementation progress and identify gaps.
- Documentation: Pre-assessment report.
- Meeting: Consultant review of audit findings.
8.2 Certification Body Selection
Task: Select Certification Body
- Description: Research and select a suitable ISO 22716 certification body.
- Documentation: Certification body selection report.
- Meeting: Final consultation to confirm the selection.
FAQ
Is this service able to help a new company achieve ISO 22716 certification?
Yes! This is not a consultancy—it’s a done-for-you solution where we handle everything for you. We don’t just advise; we write the documents, manage the process, and ensure your company meets all ISO 22716 (GMP) requirements. You get expert-crafted documentation, structured guidance, and full support until certification—without the hassle of doing it yourself.
What does unlimited requests mean?
Unlimited requests means you can ask for any type of documentation related to your ISO 22716 (GMP) certification. Whether you need procedures, quality manuals, forms, checklists, training materials, or audit documentation, we create and customize them for you—as many as you need, with no limits.
What types of documents are included in the service?
You can request any documentation needed for your ISO 22716 (GMP) certification, including:
- Quality manuals
- Standard operating procedures (SOPs)
- Forms and checklists
- Training materials
- Risk assessments
- Internal audit reports
Every document is tailored to your company’s needs to ensure full compliance with ISO 22716 Good Manufacturing Practices (GMP).
When can I expect to receive a response for my inquires?
You can expect a response to your inquiries within 12 to 24 hours. Our team ensures timely support to keep your ISO 22716 (GMP) certification process on track.
Do I have to sign a long-term contract?
No, there is no long-term contract. This is a one-time payment service with no hidden fees or recurring charges. Plus, we offer a 30-day money-back guarantee for your peace of mind.
How does the 30-day money back guarantee work?
Our 30-day money-back guarantee is simple: we work together for 30 days, and if you’re not satisfied with our work, you’ll receive a full refund—no questions asked. This ensures you can move forward with confidence, knowing there’s no risk.

Choose the Perfect Plan for Your
ISO 22716 GMP certification Journey
We offer simple, upfront pricing for ISO/IEC 17025 accreditation with no hidden costs. Choose the plan that fits your laboratory size—whether you're a small lab (1-10 personnel) or a growing facility (20-50 personnel). Our guided accreditation system ensures a smooth and efficient process—guaranteed, or you don’t pay.
Small Business Plan (1-20 Employees)
- ✅ Guaranteed Certification– Or You Don’t Pay
- ✅ Done-for-You ISO 22716 GMP Documentation
- ✅ 1-on-1 Consultant Support
- ✅ Pre-Audit Readiness Assessment
- ✅ Internal Audit & Compliance Check
- ✅ Audit-Ready Compliance Check
- ✅ Access to Your Client Portal
- ✅ Pre-Audit & Certification Audit Support
- ✅ Fast-Track Certification in 180 Days
$ 3500
Growing Business Plan (20-50 Employees)
- ✅ Everything in the Small Business Plan
- ✅ Extended 1-on-1 Support for Larger Teams
- ✅ Additional Training for Key Staff
- ✅ Custom Process Optimization
- ✅ Priority Audit Preparation & Review
$6500
Trusted by Organizations Worldwide
"See what our satisfied customers have to say about their experience with QSE Academy ISO 22716 certification system
ISO 22716 Certification – Guaranteed Quality & No-Risk Commitment
🔹 30-Day Satisfaction Guarantee – We Work, You Decide
We invest significant time and expertise upfront to craft custom documentation, guide your business, and manage your ISO 22716 (GMP) certification process. If, after 30 days of working together, you’re not satisfied with our work, we’ll issue a full refund—no questions asked. However, we don’t accept charity—this is for those serious about achieving certification.
🔹 Our Guarantee: We Work Until You Succeed
If your company follows our process but doesn’t meet ISO 22716 certification requirements on the first attempt, we continue working with you at no extra cost until you achieve compliance.
🔹 Transparent Pricing, No Hidden Fees
Our pricing is clear and upfront, with no unexpected costs. You know exactly what you’re paying for before we begin.
With our expert guidance, structured process, and commitment to results, we ensure your ISO 22716 certification—without risk or hassle.
Contact us
Have more questions? Book Your Free 30-Minute Consultation!
Struggling with ISO 22716 (GMP) certification? Not sure where to start or what’s missing in your compliance process?
Our team is offering a free 30-minute strategy call to help you:
✅ Assess your company’s current compliance status and identify key gaps.
✅ Understand ISO 22716 requirements and what they mean for your manufacturing process.
✅ Get a clear, actionable roadmap to move forward with certification.
📅 Limited slots available each week – Book your free consultation now and take the next step toward ISO 22716 compliance!


