QHSE Complete Package
2025-03-27 12:18QHSE Complete Package
Get your accreditation at the lowest possible cost


QHSE complete package
2015 version



Price : 489 $
Certification Made Simple and Accessible for Your Business
The complete QHSE (Quality, Health, Safety, and Environmental) management system package is a comprehensive document package that contains everything from templates of procedures, processes, forms, checklists, tools, detailed guides, and instructions needed to:
- Start your QHSE management system process.
- Create your QHSE documentation.
- Quickly achieve QHSE certification.
- Benefit from a QHSE management system that is simple and adapted to the needs of your organization.

Why start with a blank page. Start your Project TODAY, and save up to 80% on your time and money.

This package comes with 1 hour Live 1-to-1 Online Session with ISO consultant, document reviews, continual email support for 12 months and regular update service.

Cost-Effective Implementation: Much cheaper than an on-site consultant, and requires much less time than doing it from scratch
ISO/IEC QHSE 2015 Version Complete Package
• Added Value: All QHSE management system requirements have been developed into an efficient process that adds operational value to your organization and consequently increases productivity.
• Effective: Minimal effort is required to follow the procedures necessary to meet all QHSE requirements.
• Simplified: Bureaucracy and excessive paperwork have been eliminated from each process to make it easy—while remaining fully compliant with QHSE standards.
Start your Project TODAY, and save up to 80% on your time and money.
The all-in-one document package for QHSE 2015 version
Save time, save money and simplify the accreditation process.
Documents included:

Forms
This package provides you with the following features:
- Full lifetime access
- Access on a laptop, desktop, and mobile
Certificate of completion
This Package Includes
Procedures:
- IMS Management System Procedure
- Document Control Procedure
- Record Control Procedure
- Internal Audit Procedure
- Management Review Procedure
- Nonconformity and Corrective Action Procedure
- Risk and Opportunities Management Procedure
- Training and Competency Procedure
- Emergency Preparedness and Response Procedure
- Communication and Consultation Procedure
- Incident Investigation Procedure
- Calibration and Maintenance Procedure
- Waste Management Procedure
- Change Management Procedure
- Contractor Management Procedure
- Legal Compliance Procedure
- Performance Evaluation Procedure
Records and Forms:
- IMS Objectives and Targets Record
- Internal Audit Schedule
- Internal Audit Report Form
- Management Review Meeting Minutes
- Nonconformity and Corrective Action Request Form
- Risk Assessment Matrix
- Training Needs Analysis Form
- Training Attendance Record
- Employee Competency Assessment Form
- Emergency Drill Record
- Incident Report Form
- Calibration Record
- Maintenance Record
- Waste Disposal Record
- Change Request Form
- Contractor Evaluation Form
- Legal Compliance Register
- Performance Evaluation Results

Manual and quality policy
- Integrated Management System (IMS) Manual
Others:
- IMS Policy Statement
- IMS Organization Chart
- IMS Responsibilities and Authorities Matrix
- Process Flowcharts
- Performance Monitoring and Measurement Plan

SOPs
- SOP for Hazard Identification and Risk Assessment
- SOP for Personal Protective Equipment
- SOP for Confined Space Entry
- SOP for Lockout/Tagout
- SOP for Working at Heights
- SOP for Hot Work Permit
- SOP for Electrical Safety
- SOP for Chemical Handling and Storage
- SOP for Fire Prevention and Protection
- SOP for First Aid and Medical Emergency Response
- SOP for Environmental Aspect and Impact Assessment
- SOP for Energy Management
- SOP for Noise Control and Hearing Conservation
- SOP for Workplace Ergonomics
- SOP for Housekeeping and Sanitation
- SOP for Equipment Inspection and Testing
- SOP for Incident Reporting and Investigation
- SOP for Spill Prevention and Response
- SOP for Emergency Response and Evacuation

QHSE Documentation Requirements Explained
The QHSE Documentation Package is a vital resource for organizations looking to integrate and manage Quality, Health, Safety, and Environmental requirements into a single, cohesive management system. This comprehensive package includes all the essential documentation needed to establish, implement, and maintain a compliant and effective QHSE Management System, aligned with standards such as ISO 9001, ISO 14001, and ISO 45001.
Structured and well-maintained documentation is the foundation for delivering consistent quality, protecting employee well-being, reducing environmental impact, and maintaining regulatory compliance. From risk assessments to audit reports, this package ensures operational excellence across all four pillars.
Why QHSE Documentation Matters
An integrated QHSE system helps organizations streamline processes, reduce duplication, and maintain high standards across quality, safety, environmental, and health domains. Proper documentation ensures clarity, accountability, legal compliance, and continuous improvement while enhancing reputation and stakeholder trust.
This package includes:
Key QHSE Documentation Categories
| Document | Purpose in QHSE Management System |
|---|---|
| QHSE Policy Statement | Demonstrates top management’s commitment to quality, safety, health, and environment |
| Risk Assessment and Control Procedures | Identifies and manages risks across all QHSE domains |
| Legal and Compliance Register | Lists applicable laws, regulations, and obligations for quality, safety, and environment |
| Incident Reporting and Investigation Logs | Tracks health and safety incidents and identifies root causes |
| Environmental Impact Assessment Records | Documents effects of organizational activities on the environment |
| Quality Objectives and Performance KPIs | Measures success in achieving quality and process efficiency goals |
| Internal Audit and Corrective Action Reports | Ensures system effectiveness and continuous improvement |
| Training and Competency Records | Confirms employees are qualified, informed, and capable in their roles |
Core QHSE Documentation Requirements
Integrated Management System Documentation
To align with QHSE principles and international standards, organizations must implement a unified and systematic approach. This package includes:
QHSE Manual – Describes the structure, scope, and interaction of processes within the integrated system.
Policies and Standard Operating Procedures (SOPs) – Define specific actions related to quality, safety, environmental controls, and occupational health.
Roles and Responsibilities Matrix – Clarifies organizational structure and responsibilities across all QHSE functions.
Change Management Procedures – Manages changes in operations, systems, or infrastructure that may impact QHSE performance.
Document and Record Control Procedures – Ensures all documents are reviewed, approved, updated, and accessible when needed.
Operational Control and Risk Management Documentation
Robust controls and proactive risk management are key to an effective QHSE system. This package includes:
Job Safety Analysis (JSA) and Safe Work Method Statements (SWMS) – Identifies risks and outlines safe work practices.
Emergency Preparedness and Response Plans – Ensures readiness for fires, spills, natural disasters, or workplace incidents.
Process Monitoring and Inspection Checklists – Evaluates performance against QHSE targets and legal requirements.
Waste Management and Emissions Monitoring Logs – Tracks environmental impact and supports sustainability goals.
Preventive Maintenance Records – Keeps facilities, tools, and equipment in safe and operational condition.
Monitoring, Evaluation, and Improvement Documentation
Ongoing review and improvement are fundamental QHSE requirements. This package includes:
Internal Audit Programs and Reports – Reviews performance and identifies areas for system optimization.
Management Review Meeting Minutes – Demonstrates leadership evaluation and strategic oversight of QHSE performance.
Corrective and Preventive Action Records (CAPA) – Addresses identified non-conformities and prevents recurrence.
Customer Feedback and Complaint Handling Logs – Tracks service quality and response to external input.
Performance Metrics and Dashboards – Measures progress against quality, safety, and environmental goals.
Legal, Regulatory, and Supporting Documentation
Regulatory Compliance and Permit Tracker – Maintains visibility of environmental, health, and safety compliance obligations.
Contractor and Supplier Evaluation Forms – Ensures third parties align with your QHSE expectations.
PPE Records and Equipment Issuance Logs – Ensures personnel are properly equipped and trained.
Health Surveillance and Medical Check Records – Supports occupational health risk control.
Incident Statistics and Trend Analysis Reports – Helps identify patterns and improve preventive strategies.
Ensure Compliance with QHSE Requirements Today!
Implementing and maintaining an integrated QHSE system doesn’t have to be overwhelming. With the QHSE Documentation Package, your organization will be equipped with all the tools, templates, and procedures needed to protect people, preserve the environment, and deliver quality—safely and responsibly.
💡 Get started today and achieve operational excellence with a structured, efficient, and fully compliant QHSE documentation system!
90 Days Money Back Guarantee

If for whatever reason during the FIRST 90 days of your purchase, you are not satisfied for any reason, simply contact support@qse-academy.com and our support team will issue you an immediate and full refund.
The package includes all the documents you need to comply with QHSE 2015 – these documents are fully acceptable by the accreditation audit.
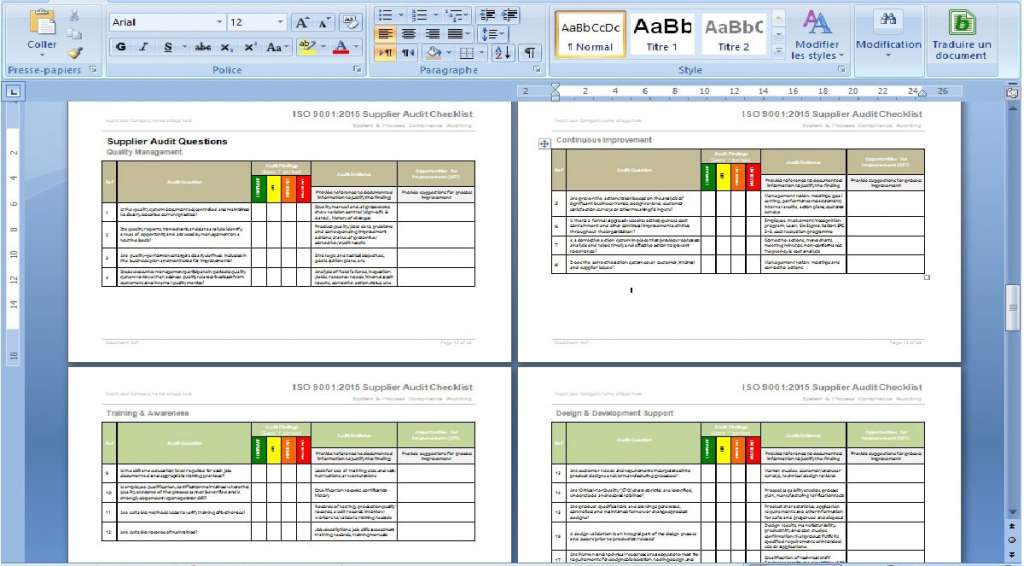
All documents are in MS Word or MS Excel, to make them very easy to customize for your business. You can customize them by adding company logos and colors, and edit headers and footers to match your favorite style.

We have already completed about 90% of the information requested on the documents. To complete them you must fill in only the name of the company, the responsible parties, and any other information unique to your company. you will be guided through the process, commenting on the elements that are needed and those that are optional.
We presented the QHSE documentation, so as to assure all its users that they have completed everything accurately and with the utmost efficiency.

All the documents are made so that you can follow the proposed order perfectly, which allows you to make sure that nothing is missing, and that no one gets lost in the process.
The included comments and flowcharts help your staff understand each document and its usefulness, which helps you to make quality management more fluid, and processes easier to follow.
Features of the complete QHSE 2015 Kit
Price: 489 $
– Documentation included: 58 documents for the implementation of IQHSE
– MS Office 2007 format, MS Office 2010, MS Office 2013
– Language: English
– Documents are fully editable – just enter the information specific to your business.
– Acceptable for the QHSE 2015 accreditation audit? Yes, all the documents required by QHSE 2015 are included, as well as the quality policy and the current but optional procedures.
Instant Delivery – The package is downloadable immediately after purchase
Free Consultation – In addition, you can submit two complete documents for review by professionals.
Created for your business – The models are optimized for small and medium businesses.


Complete QHSE 2015 Package
The complete kit to implement QHSE
Price : 489 $
Total Implementation Duration: 8 Months
QHSE Implementation Project Plan
Achieving QHSE certification is a significant milestone for any organization, signifying a commitment to quality, health, safety, and environmental standards. Our expert consultants are here to guide you through every step of the implementation process, from initial consultation and gap analysis to final assessment and certification. With our comprehensive project plan, tailored training programs, and dedicated support, we ensure your organization meets all QHSE requirements efficiently and effectively. Partner with us to enhance your credibility, improve operational processes, and gain international recognition. Let us help you achieve excellence in integrated management systems.
Introduction: Project Kick-off and Gap Analysis (Duration: 1 Month)
Introductory Tasks
1.1 QHSE Kick-off and Awareness
Task: Organize Kick-off Meeting
- Description: Conduct a kick-off meeting to introduce the QHSE management system project to key stakeholders. Discuss objectives, scope, timelines, and responsibilities.
- Deliverables: Project plan, meeting agenda, and minutes.
- Meeting: Initial consultation with senior management, QHSE coordinators, and implementation team.
1.2 Perform Gap Analysis
Task: Conduct Gap Analysis Against QHSE Standards (ISO 9001, ISO 14001, ISO 45001)
- Description: Assess current processes, management systems, and operations against relevant QHSE standards (ISO 9001 for Quality, ISO 14001 for Environment, and ISO 45001 for Health & Safety) to identify gaps.
- Deliverables: Gap analysis report with identified non-conformities.
- Meeting: Present findings to senior management and QHSE teams.
Section 1: Policy Development and Documentation (Duration: 2 Months)
2.1 Develop and Document QHSE Policy and Objectives
Task: Define QHSE Policy and Objectives (Aligning with ISO 9001, ISO 14001, ISO 45001)
- Description: Develop the organization’s integrated QHSE policy and set measurable objectives related to quality, environmental protection, and worker health & safety.
- Deliverables: QHSE policy and objectives document.
- Meeting: Review and approve the policy with senior management.
2.2 Develop Integrated Management System Documentation
Task: Create QHSE Documentation (Manual, Procedures, Work Instructions)
- Description: Develop necessary documentation for the QHSE system, including the integrated management system (IMS) manual, process procedures, and work instructions covering all aspects of QHSE.
- Deliverables: QHSE manual, procedures, work instructions.
- Meeting: Review QHSE documentation with the quality, safety, environmental, and technical teams.
Section 2: Risk Management and Legal Compliance (Duration: 2 Months)
3.1 Conduct Risk Assessment and Hazard Identification (ISO 9001, ISO 45001, ISO 14001 Clauses)
Task: Perform Risk and Environmental Aspect Assessment
- Description: Conduct a comprehensive risk assessment for health & safety hazards, quality issues, and environmental aspects across operations.
- Deliverables: Risk assessment report, hazard identification, and environmental aspect register.
- Meeting: Review risk and aspect assessment with QHSE team and department heads.
3.2 Ensure Compliance with Legal and Regulatory Requirements
Task: Identify and Monitor Legal Requirements for QHSE Compliance
- Description: Identify relevant legal, regulatory, and compliance requirements for health, safety, environment, and quality. Establish processes for monitoring compliance.
- Deliverables: Legal and compliance register, monitoring reports.
- Meeting: Review legal requirements with QHSE officers and legal teams.
Section 3: Operational Control and Emergency Preparedness(Duration: 1 Month)
4.1 Develop Operational Control Procedures (ISO 9001, ISO 45001, ISO 14001 Clauses)
Task: Establish Operational Control for QHSE Risks
- Description: Implement operational control procedures for managing quality risks, worker safety hazards, and environmental impacts. This includes equipment safety, process monitoring, and environmental protection measures.
- Deliverables: Operational control procedures and work instructions.
- Meeting: Train staff and supervisors on operational controls, QHSE responsibilities, and safety protocols.
4.2 Develop Emergency Preparedness and Response Plans (ISO 45001 and ISO 14001 Clauses)
Task: Implement Emergency Response and Incident Management Plans
- Description: Establish emergency response plans for health & safety incidents, environmental spills, and quality defects. Conduct drills and provide training.
- Deliverables: Emergency response plan, drill schedules, and incident management procedures.
- Meeting: Review and test emergency plans with QHSE and management teams.
Section 4:Competence, Training, and Worker Participation (Duration: 1 Month)
5.1 Ensure Worker Participation and Consultation (ISO 45001 Clause 5.4)
Task: Implement Worker Participation and Consultation Framework
- Description: Establish processes for consulting with and involving workers in QHSE matters, including risk identification, safety measures, and quality improvement activities.
- Deliverables: Worker participation procedures and meeting records.
- Meeting: Conduct worker participation meetings and gather input for improving QHSE initiatives.
5.2 Develop QHSE Training Program (ISO 9001, ISO 14001, ISO 45001 Clauses)
Task: Establish Training Programs for Employees and Contractors
- Description: Develop and implement a training program to ensure all personnel are aware of QHSE risks and the necessary safety and quality procedures.
- Deliverables: Training plans, training materials, and attendance records.
- Meeting: Conduct QHSE training sessions and workshops for employees and contractors.
Section 5: Monitoring, Measurement, and Incident Management (Duration: 1 Month)
6.1 Monitor QHSE Performance (ISO 9001, ISO 14001, ISO 45001 Clauses)
Task: Develop Monitoring and Performance Measurement Processes
- Description: Implement processes to monitor and measure the effectiveness of the QHSE system, including tracking key performance indicators (KPIs) for quality, safety, and environmental protection.
- Deliverables: QHSE performance reports, monitoring tools, and dashboards.
- Meeting: Monthly performance review meetings with QHSE team.
6.2 Implement Incident Investigation and Corrective Action Procedures (ISO 45001, ISO 9001 Clauses)
Task: Develop Procedures for Incident Reporting and Root Cause Analysis
- Description: Establish a procedure for investigating QHSE incidents (such as safety incidents, environmental spills, or quality failures), including root cause analysis and corrective actions.
- Deliverables: Incident investigation reports, root cause analysis documents, corrective action plans.
- Meeting: Review incidents and corrective actions with QHSE and department heads.
Section 6: Internal Audits and Management Review (Duration: 1 Month)
7.1 Develop Internal Audit Program (ISO 9001, ISO 14001, ISO 45001 Clauses)
Task: Create Internal Audit Plan
- Description: Develop an internal audit program to regularly assess the effectiveness of the integrated QHSE management system and compliance with relevant ISO standards.
- Deliverables: Internal audit plan, audit schedule, and checklist.
- Meeting: Review audit plan with internal auditors and senior management.
7.2 Conduct Internal Audits
Task: Perform Internal Audits
- Description: Conduct internal audits to evaluate the QHSE system’s compliance with ISO standards, identifying non-conformities and areas for improvement.
- Deliverables: Internal audit reports, non-conformance reports.
- Meeting: Post-audit review meeting to discuss findings and corrective actions.
7.3 Conduct Management Review (ISO 9001, ISO 14001, ISO 45001 Clauses)
Task: Perform Management Review of QHSE System
- Description: Conduct a management review to evaluate the performance of the QHSE system, assessing audit results, process effectiveness, and opportunities for improvement.
- Deliverables: Management review minutes, action plans.
- Meeting: Present findings and improvement plans to senior management.
Final Assessment: Certification Preparation and External Audit (Duration: 1 Month)
8.1 Conduct Pre-Certification Internal Audit
Task: Perform Pre-Certification Internal Audit
- Description: Conduct a final internal audit to ensure that the QHSE system meets ISO 9001, ISO 14001, and ISO 45001 requirements and is ready for certification audits.
- Deliverables: Pre-certification audit report, corrective action plans.
- Meeting: Final review meeting with senior management and the QHSE team.
8.2 Certification Body Selection and External Audit
Task: Select Certification Body and Schedule Certification Audit
- Description: Research and select an accredited certification body for ISO 9001 (Quality), ISO 14001 (Environment), and ISO 45001 (Health & Safety). Schedule the external audit and ensure the organization is fully prepared.
- Deliverables: Certification body selection report, external audit schedule.
- Meeting: Final meeting with management and QHSE teams to confirm readiness for certification.
This 8-month project plan for QHSE (Quality, Health, Safety, and Environment) management system implementation ensures a structured approach to achieving compliance and certification for integrated quality, health & safety, and environmental standards. It covers key areas such as risk management, operational controls, worker participation, internal audits, and continual improvement, aligning the organization with ISO 9001, ISO 14001, and ISO 45001 standards.
What our customers think:

“The QHSE Complete Package from QSE Academy provided everything we needed to implement a robust management system. The pre-made templates and documents helped us fast-track our compliance without wasting time on creating materials from scratch. The guidance from the one-on-one sessions was extremely helpful, making the process much smoother. This package significantly reduced our consulting expenses and allowed us to focus on other areas of our business. Highly recommended for any organization looking to achieve QHSE accreditation efficiently!”
Rachel Moore
Health & Safety Manager


“We were looking for an affordable solution to implement our QHSE management system, and the QHSE Complete Package from QSE Academy was perfect for our needs. It came with comprehensive, customizable templates that saved us a lot of time and effort. The detailed instructions made it easy to follow, and the continuous support from their team ensured we were on track. This package saved us both time and money compared to hiring full-time consultants. It’s one of the best investments we’ve made for our company.”
John Carter
Operations Director

Frequently Asked Questions
How long will it take to receive the complete package of documents after I place my order?
Upon completing your purchase, you will be redirected to the download page immediately. Additionally, a link to access your file will be sent to your email. The files are provided in a .zip format, which you will need to extract. If you encounter any issues with the download, please do not hesitate to contact us at support@qse-academy.com. Our support team is always ready to assist you.
What payment methods can I use?
We offer several payment options for your convenience. You can choose to pay using a credit card, debit card, or PayPal. Additionally, we provide a flexible layaway plan for those who prefer to pay for their purchase over time. If you have any questions about our payment options, please don’t hesitate to contact us.
Do you offer a money-back guarantee if I'm not satisfied with the service?
We offer a 30-day money-back guarantee. If you are not satisfied with our service for any reason, you can cancel within the first 30 days and receive a full refund, no questions asked.
Is there ongoing support or assistance available after my purchase?
Yes! At QSE Academy, our ISO experts provide continued support by answering your queries via email. You can expect a detailed response within 24 to 48 hours to help you move forward confidently.
Are updates to the documentation package included after purchase?
Absolutely. To ensure your documentation remains reliable and compliant, we update our packages every 6 months. Existing customers receive these minor updates at no extra charge. However, when there’s a major revision of the ISO standard itself, you’ll need to purchase an updated kit to align with the new standard.
Will I receive a valid invoice for my business expenses after completing the purchase?
Yes. After completing your purchase, you’ll immediately receive a valid invoice suitable for business and tax purposes. If you require any specific adjustments or details added to your invoice, please reach out to our support team.
Can I customize these documents for my company's specific needs?
Yes, the documents are fully customizable! You can easily edit, modify, and add your company’s logo to tailor them specifically for your organization. Additionally, if you’d prefer assistance, we offer a personalized “Done-For-You” customization service to deliver audit-ready documents tailored exactly to your organization’s requirements.
How quickly can I implement this ISO standard using your documentation?
Implementation time varies depending on your company’s engagement, resources, and experience. Typically, we’ve observed businesses successfully achieve compliance and certification within 3 to 6 months using our clear, structured documentation packages.
Do these documents guarantee successful certification?
While our documentation packages significantly simplify the certification process, the ultimate success of ISO certification depends on effective implementation. For organizations seeking further assurance, we also provide comprehensive support services, including guided implementation and internal audits, to help you confidently pass your certification audit.
Do you offer hands-on assistance if I need extra help during implementation?
Definitely! If you prefer a complete, hands-off solution, we offer a premium “Done-For-You” implementation service. Our ISO experts handle the full preparation, providing you with audit-ready documentation and detailed implementation support. You simply adopt the customized materials, follow the tailored guidelines, and confidently pass your audit.






