Quality Manual for ISO/IEC 17021 2015
The creation of the ISO/IEC 17021 Quality Manual reflects an organization’s commitment to upholding the highest conformity assessment standards. ISO/IEC 17021:2015 sets the criteria for certification bodies providing audits and certifications of management systems. It is the foundation of trust in international trade and assures that these bodies are competent and impartial.
简介
ISO/IEC 17021:2015 stands as a pivotal standard for certification bodies involved in auditing and certifying different management systems. The standard delineates requirements intended to ensure that these organizations offer services in a manner that is both consistent and impartial, while also maintaining a high level of technical competency. The pertinence of ISO/IEC 17021:2015 spans across a multitude of management systems standards, from quality and environmental to security management systems.
The creation and implementation of a Quality Manual in accordance to ISO/IEC 17021 are crucial for certification bodies. A Quality Manual acts as a testament to the organization’s commitment to integrity, outlining its standard operating procedures, mandatory procedures, and policies. Additionally, it assists in elucidating the structure of the management system, providing a clear roadmap for both internal governance and adherence to accreditation rules.
In this article, readers will gain insights into the prime importance of a Quality Manual for certification bodies and how it underpins the effectiveness of audit and certification activities. Understanding its significance is essential for these bodies not only to achieve but also to maintain compliance with the stringent requirements of ISO/IEC 17021:2015, thereby assuring their clients of the quality and reliability of the certifications awarded.
Understanding the Role of a ISO/IEC 17021 Quality manual
The Quality Manual plays a crucial role within the framework of ISO/IEC 17021, the standard for certification bodies issuing certificates for various types of management systems. This manual serves as the cornerstone document, delineating the procedures and protocols certification bodies must adhere to for accrediting management systems.
A well-crafted Quality Manual goes beyond mere documentation; it underscores the certification body’s commitment to maintaining the technical competence and impartiality required for third-party assessments. Furthermore, it fosters an atmosphere of trust amongst clients and stakeholders by showcasing the certification body’s dedication to upholding the highest standards of quality management.
In the context of ISO/IEC 17021:2015, the Quality Manual is beneficial because it heightens transparency and consistency across certification activities. Through clear guidelines and standard operating procedures, it ensures uniformity in executing certification tasks—be it audit checklists, analysis of external documentation, or managing special audits.
The essential components of a quality manual include the scope of certification activities, details of the processes and their interactions, the policy for maintaining impartiality, descriptions of roles and responsibilities, and procedures for managing records and documents. A well-structured manual will typically provide sample formats and blank forms to streamline processes.
By embracing the principles outlined in the ISO/IEC 17021 Quality Manual, certification bodies effectively convey their professionalism and reliability, paving the way for enhanced credibility in the marketplace. The manual is more than a set of instructions—it is a reflection of the certification body’s identity and its unwavering dedication to quality and service excellence.
Initial Steps in Developing an ISO/IEC 17021 Quality manual
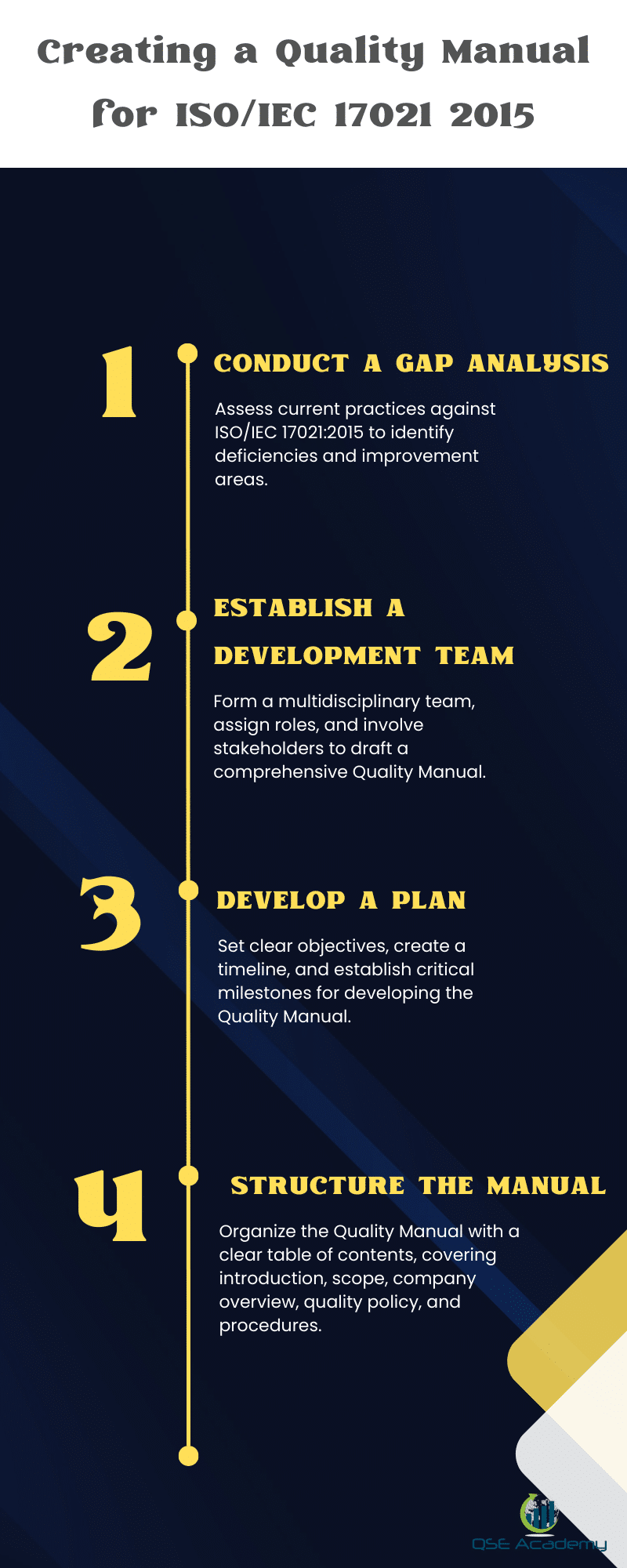
When embarking on the development of a Quality Manual in compliance with ISO/IEC 17021 requirements, the initial steps are crucial for a successful outcome. The two prominent initial stages in this process involve conducting a gap analysis and then establishing a development team.
Conducting a Gap Analysis: This phase is instrumental in assessing current practices and documentation against the ISO/IEC 17021 standard. The gap analysis enables certification bodies to pinpoint deficiencies and potential areas for enhancement within their management systems. The methodology involves a systematic comparison which yields critical data for shaping the Quality Manual.
Establishing a Development Team: The creation of a dedicated Development Team is the next strategic move. Bringing together key personnel and stakeholders ensures a cross-functional approach to Quality Manual development. Each team member is allocated specific roles and responsibilities, fostering a collaborative environment necessary for drafting a comprehensive manual.
Developing a Plan: With insights from the gap analysis and a competent team in place, setting clear objectives is the next course of action. Developing a plan that outlines the goals, the timeline, and the critical milestones establishes an actionable roadmap toward achieving a robust Quality Manual.
Initial Steps in Quick Reference:
- Assess current systems against ISO/IEC 17021
- Identify discrepancies and improvement opportunities
- Form a multidisciplinary Development Team
- Assign clear roles and involve stakeholders
- Draft an objective-driven plan with established timelines
These foundational moves pave the path towards a structured and compliant Quality Manual that embodies the standard operating procedures, mandatory procedures, and reflects the integrity and technical competence expected of certification bodies.
Structuring the ISO/IEC 17021 Quality manual
The Quality Manual is a structural cornerstone for certification bodies operating under ISO/IEC 17021, which sets out requirements for bodies providing audit and certification of management systems. Ensuring that the manual is structured correctly is pivotal, as it guides certification activities, ensuring the technical competence and impartiality of the certification process while aligning with the stringent accreditation rules.
A well-structured Quality Manual begins with a definitive Table of Contents, facilitating ease of navigation and quick access to crucial information. A sample table of contents might include sections on the introduction and scope of the manual, a comprehensive overview of the certification body, and in-depth discussion on the company’s quality policy, detailing its commitment to upholding standards and dedication to continuous improvement.
The introduction clarates the manual’s purpose and outlines the procedural framework for certification activities, while the scope delineates the types of management systems the body assesses, from quality management to security management systems.
A succinct company overview provides a snapshot of the body’s mission, vision, and values, reaffirming its pledge to impartiality and service excellence.
The quality policy underscores the organization’s allegiance to the highest quality standards and objectives that equip it to conduct unbiased and competent third-party assessments.
To ensure completeness, subsequent sections must encapsulate mandatory procedures, standard operating procedures for audit and certification activities including special audits, and the management of external documentation. These segments should include clear instructions, sample formats, audit checklists, and blank forms – a suite of tools essential for upholding the ISO/IEC 17021:2011 requirements.
The quality manual, as a living document, should evolve over time to incorporate changes and improvements in procedures, reflecting the dynamic nature of the certification body’s operations. It’s the blueprint that outlines the adherence to accreditation standards set out by bodies such as ANAB (ANSI National Accreditation Board) and secures the organization’s status as a credible certification body.
Documenting Procedures
Documenting procedures is an integral part of the ISO/IEC 17021 standard, ensuring consistency in certification activities and maintaining the integrity and impartiality of certification bodies. Below are essential procedures that certification bodies should meticulously document:
文件控制程序
To safeguard the credibility of certification processes, a robust document control procedure must be in place. This includes:
- Methodical creation, approval, and revision of documents.
- Maintaining documents in a manner that ensures they are current and accessible to relevant parties.
审计程序
Effective audits are the cornerstone of certification. Documenting this procedure involves:
- Detailed planning and execution of audits.
- Recording of audit findings, followed by appropriate corrective actions.
认证决定程序
A transparent certification decision process is critical for maintaining trust in the ISO/IEC 17021 system. It includes:
- Thorough documentation of certification decisions.
- Ensuring decisions are made with competence and impartiality, void of conflicts of interest.
投诉和上诉程序
A process that demonstrates commitment to fairness and responsiveness involves:
- Receipt and evaluation of complaints and appeals.
- Documented resolutions and communication to the involved parties.
| 程序 | Key Documentation Elements |
| 文件控制 | Approval, Revision Tracking, Accessibility |
| 审计 | Audit Plan, Findings, Corrective Actions |
| 认证决定 | Decision Rationale, Impartiality Assurance |
| 投诉和上诉 | Complaints Log, Resolution, Outcome Communication |
Certification bodies must adhere to these procedures to ensure technical competence and impartiality while providing certification services. Moreover, properly documented procedures not only reinforce the quality management principles of the certification body but also enhance transparency and trust among customers and Accreditation Bodies.
By adhering to these clear-cut procedures, certification bodies can deliver high-quality and consistent service, thereby reinforcing their position as trustworthy third-party assessors in management systems certification.
Defining Roles and Responsibilities
Defining roles and responsibilities within a certification body is a cornerstone in meeting the requirements of ISO/IEC 17021, which sets out the criteria for bodies providing audit and certification of management systems. This ensures not only the smooth operation of the certification process but also upholds the integrity and impartiality of the assessments. Here’s an overview of how a certification body structures its hierarchy and assigns duties.
组织结构
An effectively outlined organizational structure forms the backbone of a certification body. It provides clarity on reporting lines and decision-making authority, essential for maintaining impartiality and consistency in certification activities.
Top Management Responsibilities
Top management bears the ultimate responsibility for upholding the quality and compliance of certification activities. They demonstrate their commitment by:
- Ensuring the availability of necessary resources.
- Fostering a quality-conscious ethos.
- Reviewing the performance of the quality management system regularly.
Auditor and Staff Responsibilities
The role of auditors and staff is pivotal in executing the certification body’s mandate. Their responsibilities include:
- Conducting rigorous and unbiased audits.
- Maintaining technical competence and staying updated on standards and regulations.
- Adhering to defined standard operating procedures and mandatory protocols.
Roles and Responsibilities Table
| Position | Responsibilities |
| Top Management | – Quality and compliance commitment
– Resource provision – Strategic direction and performance review |
| Auditors | – Performing audits in line with ISO/IEC 17021
– Ongoing professional development – Adherence to impartiality and confidentiality guidelines |
| Support Staff | – Scheduling and coordinating audits
– Managing external and internal documentation – Assisting in the maintenance of the quality management system |
The certification body must regularly evaluate and refine the competences required for each role to ensure the highest standards are met. It involves a thorough selection criteria for auditors, comprehensive training programs, and periodic performance reviews. In doing so, the certification body solidifies its reputability and the trust of the organizations it certifies.
Managing Resources
In managing the resources critical for a certification body, the ISO/IEC 17027 standard outlines specifications to ensure effective operations. Here we delve into three fundamental areas: Human Resources, Infrastructure and Facilities, and Financial Resources.
人力资源部门
For a certification body to deliver valid and reliable services, it demands a team of adept personnel. Recruitment and selection processes are pivotal and must focus on competence, qualifications, and experience. Continuous investment in staff through training and development is not just encouraged; it is mandatory for maintaining the technical competence required by ISO/IEC 17021.
Key Points:
- Competent recruitment and selection
- Ongoing training
- Professional development
基础设施和设备
A robust infrastructure is the cornerstone of smooth certification activities. A well-maintained facility not only houses the essential operations but also signals professionalism and efficiency. Regular facility improvements highlight the commitment of a certification body to quality and adherence to the requirements of the ISO/IEC 17021 standard.
Key Points:
- Adequate and supportive infrastructure
- Regular maintenance and improvement
Financial Resources
Financial health is integral to a certification body’s endurance and capability to deliver uncompromised service. Proper financial planning and budgeting must be in place to secure the company’s fiscal steadiness. This ensures sustained operations, even when the market ebbs and flows, aligning with the imperative of long-term sustainability as demanded by ISO/IEC 17021.
Key Points:
- Thoughtful financial planning
- Ensuring financial sustainability
In conclusion, these resource management principles are critical for the successful functioning and credibility of any certification body. They help maintain trust and integrity in the certification activities carried out under the ISO/IEC 17021 standard, paving the way for impartial and technically competent service delivery.
Implementing Quality Control
Quality control is crucial for certification bodies to ensure adherence to the ISO/IEC 17021 standard. This standard sets the benchmark for organizations that provide certification of management systems, aiming for consistency and impartiality in their certification activities. To maintain high standards, a certification body must integrate rigorous quality control processes into their operation.
内部审计: Essential for quality control, internal audits allow a certification body to self-examine and catch potential non-conformities. By diligently conducting these audits, the organization ensures its processes are always aligned with ISO/IEC 17021 requirements. Audits need to be systematic, so an audit checklist and procedures should be integral components of the Quality Manual.
管理评论: Performed regularly, management reviews are a strategic tool for senior leadership to scrutinize the effectiveness of the Quality Management System (QMS). These reviews facilitate continual improvement by addressing any identified issues and adapting strategies to enhance services.
Monitoring and Measurement: Objectives need to be measurable. Performance metrics offer a clear view of progress towards these objectives. By monitoring and tracking performance, the organization can not only ensure compliance with standards but also focus on areas that require improvement. The Quality Manual should include methodologies to measure and monitor these metrics.
These measures serve as pillars of robust quality control. Quality manuals need to outline these practices clearly, providing staff with the necessary guidance to perform their certification tasks efficiently and impartially. Implementing quality control not only ensures the technical competence of the certification body but also supports the credibility and value of the certifications it provides.
| Quality Control Component | Function |
| 内部审计 | Ensure ongoing compliance with ISO/IEC 17021 |
| 管理评论 | Drive improvement and maintain standards |
| Monitoring and Measurement | Evaluate performance against set metrics |
These components help maintain the integrity of certification activities, ensuring clientele receive a consistent, reliable, and impartial service.
持续改进
In the ISO/IEC 17021 Quality Manual, Continuous Improvement stands as a cornerstone principle, driving certification bodies to excel in their certification activities and services. This section delineates the strategic approach to refining processes and enhancing customer satisfaction.
Certification bodies should set clear objectives focused on perpetual enhancement. By implementing corrective and preventive measures in response to internal audits, management reviews, or feedback, bodies can mitigate nonconformities and advance their services.
Feedback mechanisms are vital in this improvement loop. Gathering insights from clients and stakeholders, certification bodies can pinpoint areas for enhancement. Utilizing both positive and negative feedback, they can make informed decisions that lead to improved processes and more efficient certification activities.
Recording improvements is as crucial as making them. Changes to the quality manual must be meticulously documented, providing a clear audit trail of adjustments and their rationales. Effective communication of these changes ensures that all personnel understand and adopt the revised procedures.
A sample format for documenting improvements might include a table with columns for the date, nature of the improvement, reason for the change, and the implementing individual or team. Ensuring these updates are properly managed will exemplify the technical competence and commitment to quality that the ISO/IEC 17021 standard expects from certification bodies.
Continuous improvement is not just a requirement but a commitment to excellence—a symbol of the body’s impartiality and unwavering dedication to the technical competence and value they bring to every client.
总结
In conclusion, the ISO/IEC 17021 Quality Manual is an invaluable asset for any certification body committed to excellence in certifying management systems. By adopting a comprehensive Quality Manual, certification bodies not only guarantee compliance with ISO/IEC 17021 standards, but they also enhance their credibility and reliability in the eyes of Accreditation Bodies and clients. It serves as a roadmap for certification activities, providing clear procedures, sample formats, and audit checklists, which facilitate efficient and effective certification processes.
The benefits conferred by a well-designed Quality Manual are manifold, including the establishment of mandatory and standard operating procedures that ensure impartiality and technical competence during third-party assessments. Moreover, when a Quality Manual is in place, certification bodies possess a tool that continually guides and improves quality management practices.
For these reasons, organizations should not overlook the importance of a Quality Manual in their pursuit of quality excellence. Emphasis should be placed not only on the initial document preparation, which often involves cost, but also on the ongoing role the manual plays in upholding the essence of quality management. The Quality Manual is a living document, integral to fostering improved service delivery and strengthening trust between certification bodies and their money-to-customers.
寻找有关 ISO 17021 的更多资源?
如果本文对您有所帮助,请浏览我们旨在帮助您高效获得 ISO 17020 认证的优质资源:
- 📦 ISO/IEC 17021-1 2015 完整文件包: 获取快速、轻松实施所需的所有基本模板和文件。
- 🎓 ISO/IEC 17021-1 2015 在线课程 : 参加我们的综合培训,掌握获得认证的关键概念和实际步骤。
- 📋 ISO/IEC 17021-1 2015 检查表: 下载我们的详细核对表,确保您已完成流程的每个步骤。
这些资源可满足您的需求,确保您顺利通过认证。 今天就来探索它们,离成功更近一步!